1. Introduction
Overview of Potrate and Its Therapeutic Classification
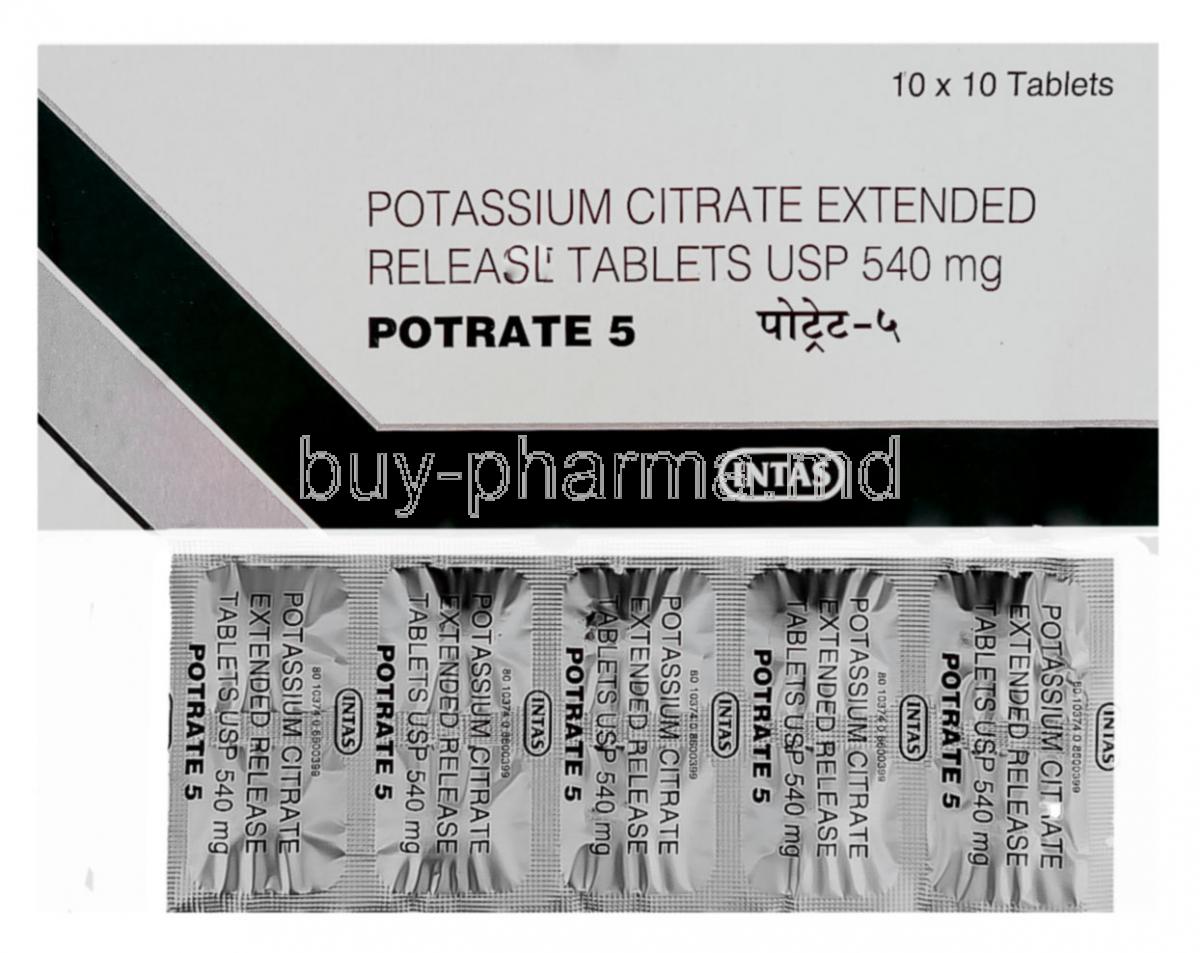
Potrate, a formulation of Potassium Citrate Extended-Release, is a therapeutic agent classified as a urinary alkalinizer. It is primarily prescribed for managing disorders related to urinary acidification and crystal formation within the kidneys. Its pharmacological purpose lies in neutralizing urinary acidity, thereby preventing the formation of kidney stones and maintaining electrolyte balance.
Medical Relevance of Potassium Citrate Extended-Release Formulation
The extended-release design of Potrate ensures a gradual and sustained release of potassium citrate into the bloodstream. This steady absorption maintains optimal urinary alkalinity over an extended period, reducing the frequency of dosing and improving patient compliance. It is particularly valuable in chronic management of nephrolithiasis and renal tubular acidosis.
Approved Status and Availability in Different Countries
Potrate is approved in multiple regions under various brand names for therapeutic use. It is available in both prescription and over-the-counter formulations depending on local regulations. In regions like Asia, Europe, and the Middle East, it is widely used in nephrology and urology practices.
Summary of Key Clinical Benefits and Patient Outcomes
- Maintains balanced urinary pH, preventing stone recurrence.
- Reduces urinary calcium and oxalate concentration.
- Improves overall renal function in metabolic acidosis.
- Enhances patient quality of life through non-invasive management.
2. Composition and Formulation
Active Ingredient: Potassium Citrate (Extended-Release)
Each Potrate tablet contains Potassium Citrate ER as the active component, formulated to release the medication gradually to ensure sustained efficacy and minimized gastrointestinal discomfort.
Inactive Excipients and Tablet Structure
The inactive excipients typically include pharmaceutical-grade binders, lubricants, and coating agents. These components stabilize the active substance and ensure smooth intestinal transit.
Mechanism of Extended-Release Delivery System
The extended-release matrix ensures controlled ion exchange over several hours. This system allows a continuous alkalinizing effect without sharp peaks in serum potassium levels, enhancing safety for patients requiring long-term therapy.
Available Strengths and Dosage Forms
Potrate is commonly available in 5 mEq, 10 mEq, and 15 mEq tablet strengths. Each tablet is film-coated for easy swallowing and protection from humidity.
3. Mechanism of Action (How Potrate Works)
Role of Potassium Citrate in Urinary Alkalinization
Potassium citrate dissociates into potassium ions and citrate ions. The citrate acts as a systemic alkalinizing agent by neutralizing hydrogen ions in the urine, elevating urinary pH levels.
Regulation of Urinary pH and Reduction of Calcium Oxalate Crystallization
By increasing urinary pH, Potrate prevents the precipitation of calcium oxalate and uric acid crystals. This process significantly lowers the risk of kidney stone formation in predisposed individuals.
Enhancement of Citrate Excretion and Prevention of Stone Formation
Citrate binds with urinary calcium, forming soluble complexes that inhibit crystallization. This mechanism underpins its clinical utility in recurrent nephrolithiasis prevention.
Systemic Effects on Acid-Base Balance
Potrate contributes to systemic buffering by reducing metabolic acidosis, promoting optimal enzyme activity and metabolic equilibrium across various organ systems.
Comparison with Other Urinary Alkalinizers and Citrate Salts
Compared to sodium citrate or bicarbonate formulations, potassium citrate avoids sodium overload, making it preferable for patients with hypertension or cardiovascular conditions.
4. Therapeutic Uses
4.1 Approved Indications
- Prevention and treatment of kidney stones (calcium oxalate, uric acid types)
- Management of renal tubular acidosis and chronic acidotic states
- Correction of hypocitraturia in metabolic disorders
- Prevention of uric acid crystallization in patients with gout or hyperuricemia
4.2 Off-Label and Adjunctive Uses
- Adjunctive therapy for chronic kidney disease to counteract metabolic acidosis
- Maintenance of urinary alkalinity in cystinuria to prevent stone formation
- Post-chemotherapy renal protection through alkalinization
- Supportive care in recurrent nephrolithiasis with dietary therapy
5. Pharmacokinetics and Pharmacodynamics
Absorption Profile of the Extended-Release Formulation
Potrate ER is absorbed slowly through the gastrointestinal tract. This minimizes gastrointestinal irritation while maintaining stable serum potassium levels for 12–24 hours.
Distribution and Plasma Concentration Curve
The compound distributes primarily within extracellular fluids, with steady-state levels achieved through continuous dosing.
Metabolism and Renal Elimination Pathway
Potassium is handled primarily through renal excretion. The citrate component undergoes hepatic metabolism into bicarbonate, contributing to alkalinization.
Duration of Action and Onset of Effects
Onset typically occurs within 1–2 hours post-ingestion, with sustained effects lasting up to 12 hours, depending on renal function and hydration status.
6. Dosage and Administration
Standard Adult Dosage for Urinary Alkalinization
Typical adult dosing ranges from 30 to 100 mEq daily, divided into two or three doses depending on urinary pH response and medical supervision.
Titration Based on Urinary pH and Citrate Levels
Dosing adjustments should be guided by urinary pH measurements. The therapeutic target is a urinary pH between 6.0 and 7.0.
Dosage Recommendations for Renal Tubular Acidosis
Patients with renal tubular acidosis generally require 60–100 mEq daily, adjusted to maintain normal plasma bicarbonate levels.
Administration Timing with or After Meals
Potrate tablets should be taken with or immediately after meals to minimize gastrointestinal upset and improve absorption.
Guidelines for Missed Doses and Dose Adjustments
If a dose is missed, it should be taken as soon as remembered unless it is close to the next scheduled dose. Doubling doses is not recommended.
Maximum Daily Dosage and Clinical Monitoring
The daily dose should not exceed 100 mEq. Serum electrolytes and renal function should be routinely assessed during prolonged therapy.
7. Important Precautions and Careful Administration
Regular Monitoring of Serum Potassium and Renal Function
Continuous monitoring is crucial to prevent hyperkalemia and ensure optimal renal performance. Electrocardiographic evaluation may be required in at-risk patients.
Adjustments for Patients with Mild Renal Impairment
Reduced dosing and extended intervals are recommended for patients with diminished renal clearance to prevent accumulation.
Avoiding Concurrent Potassium Supplements or Salt Substitutes
Simultaneous use of potassium-based products can elevate serum potassium dangerously. Such combinations should be avoided unless strictly supervised.
Guidance on Hydration and Dietary Sodium Restriction
- Maintain adequate fluid intake throughout therapy.
- Limit sodium-rich foods to enhance the drug’s alkalinizing efficiency.
Caution in Patients with Cardiac Arrhythmias or Dehydration
Patients with a history of cardiac abnormalities or dehydration should use Potrate under close medical observation to prevent adverse cardiovascular effects.
8. Contraindications
- Known hypersensitivity to potassium citrate or related compounds
- Severe renal impairment or oliguria
- Existing hyperkalemia or conditions promoting potassium retention
- Untreated Addison’s disease
- Active gastrointestinal ulcer or bleeding
9. Warnings and Clinical Monitoring
Risk of Hyperkalemia and Associated Cardiac Events
Excess potassium may induce bradycardia, arrhythmia, or cardiac arrest. Monitoring serum potassium levels is mandatory in patients on prolonged therapy.
Monitoring Requirements During Prolonged Therapy
Periodic evaluation of electrolyte panels, ECGs, and renal parameters should be performed to maintain therapeutic safety and efficacy.
Signs and Symptoms of Potassium Toxicity
- Muscle weakness and tingling sensations
- Irregular heartbeat or palpitations
- Fatigue and nausea
- In severe cases, cardiac arrest
Discontinuation Criteria and Emergency Management Guidelines
Therapy should be discontinued immediately if hyperkalemia is confirmed. Emergency management includes intravenous calcium, glucose-insulin infusions, or dialysis as appropriate.
```html
10. Drug Interactions
Interactions with Potassium-Sparing Diuretics (e.g., Spironolactone, Amiloride)
Potrate should not be administered concomitantly with potassium-sparing diuretics such as spironolactone, triamterene, or amiloride. These agents reduce urinary potassium excretion, leading to a marked risk of hyperkalemia. This can precipitate life-threatening cardiac arrhythmias or conduction abnormalities.
ACE Inhibitors and ARBs Leading to Elevated Potassium Levels
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) can elevate serum potassium by reducing aldosterone-mediated excretion. When combined with Potrate, this effect is potentiated. Routine monitoring of serum potassium and renal function is essential during concurrent therapy.
Anticholinergic and Opioid Medications Affecting GI Motility
Medications with anticholinergic or opioid properties may slow gastrointestinal transit time. When taken alongside Potrate, prolonged mucosal exposure to the drug may occur, increasing the likelihood of gastrointestinal irritation, ulceration, or obstruction.
Interaction with Aluminum-Containing Antacids
Concurrent administration with aluminum-based antacids may reduce citrate absorption and decrease its alkalinizing efficacy. It is advisable to separate administration by at least 2 hours to prevent pharmacological interference.
Laboratory Interference and Monitoring Implications
Potassium citrate may alter results of electrolyte and renal function tests. Elevated serum potassium levels may appear artificially increased if samples are hemolyzed. Regular laboratory evaluation ensures early detection of metabolic disturbances.
11. Adverse Effects
11.1 Common Side Effects
Most patients tolerate Potrate well; however, some may experience mild to moderate adverse effects, particularly during initiation. Common reactions include:
- Nausea, vomiting, and mild gastrointestinal discomfort due to localized irritation.
- Abdominal bloating and diarrhea resulting from transient osmotic effects in the GI tract.
- Temporary taste alterations or metallic aftertaste, which typically resolve spontaneously.
- Mild fatigue or muscle weakness associated with minor electrolyte fluctuations.
11.2 Serious and Rare Side Effects
Though uncommon, severe adverse events can occur with improper use or overdose:
- Hyperkalemia and cardiac conduction abnormalities—manifesting as arrhythmia, bradycardia, or cardiac arrest.
- Gastrointestinal ulceration or obstruction from tablet impaction, particularly in patients with GI motility disorders.
- Metabolic alkalosis resulting from excessive urinary alkalinization.
- Overdose toxicity characterized by muscle paralysis, irregular pulse, or syncope.
12. Administration in Special Populations
12.1 Use in Elderly Patients
In elderly individuals, diminished renal clearance increases susceptibility to hyperkalemia. Doses should be carefully titrated and renal function closely monitored. Elderly patients are also more likely to be on multiple medications, necessitating vigilance against drug-drug interactions and cumulative cardiac risks.
12.2 Use in Pregnant and Nursing Women
Available clinical and animal data suggest no significant teratogenic effects. However, potassium citrate should be prescribed during pregnancy only if the potential benefit outweighs possible risks. During lactation, small amounts of potassium may appear in breast milk, but adverse effects in nursing infants are rare. Physicians should assess the need for therapy based on maternal metabolic demands and renal status.
12.3 Use in Children
Potrate may be prescribed in pediatric populations for specific metabolic disorders under medical supervision. Dosage must be individualized based on body weight and renal capacity. Long-term administration requires periodic evaluation of growth, urinary pH, and serum electrolytes to prevent imbalances and ensure normal development.
13. Overdosage and Management
Clinical Signs of Potassium Toxicity and Hyperkalemia
Overdose may present with profound weakness, numbness, slow or irregular heart rate, or cardiac arrest. Elevated serum potassium levels exceeding 6.5 mEq/L signify severe toxicity.
Immediate Emergency Measures
- Initiate cardiac monitoring immediately.
- Administer intravenous calcium gluconate to stabilize cardiac membranes.
- Infuse insulin with glucose to promote intracellular potassium uptake.
- Use beta-agonists or sodium bicarbonate when indicated.
Role of Dialysis in Severe Cases
In patients with renal failure or refractory hyperkalemia, hemodialysis remains the most effective method for potassium removal and metabolic correction.
Prevention Strategies for Accidental Overdose
Patients should avoid self-adjusting doses and refrain from combining Potrate with other potassium sources. Proper labeling and secure storage reduce the risk of accidental ingestion, especially in children or the elderly.
14. Handling and Storage
Recommended Storage Temperature and Humidity Conditions
Potrate tablets should be stored at a controlled room temperature of below 30°C (86°F), with humidity levels maintained under 60%. Avoid exposure to direct sunlight.
Protection from Light and Moisture
The tablets should be kept in their original, tightly sealed containers to prevent degradation. Moisture can alter the tablet’s integrity and release profile.
Safe Handling and Disposal of Expired Tablets
Expired tablets should not be disposed of through household waste or wastewater. Return them to a designated pharmacy collection point for safe destruction.
Guidelines for Transport and Repackaging
During transport, ensure tablets remain protected from vibration and temperature extremes. Repackaging should be conducted in moisture-proof blister packs to preserve product stability.
15. Patient Counseling Information
Importance of Adherence to Prescribed Dose
Patients must adhere strictly to the dosing schedule prescribed by their healthcare provider. Skipping or doubling doses can compromise therapeutic control or increase the risk of hyperkalemia.
Dietary Recommendations to Maintain Urinary pH Balance
- Increase intake of water and fruits rich in natural citrate, such as lemons or oranges.
- Avoid high-sodium foods and excessive protein, which can acidify urine.
- Refrain from using salt substitutes containing potassium chloride.
Recognizing Symptoms of Electrolyte Imbalance
Patients should promptly report warning signs such as muscle cramps, palpitations, dizziness, or persistent nausea. Early recognition prevents serious complications.
Regular Follow-Up and Laboratory Monitoring Schedule
Periodic laboratory evaluations—typically every 4 to 8 weeks during long-term use—are recommended to monitor serum electrolytes, renal function, and urinary pH levels. Adherence to follow-up appointments ensures both efficacy and safety throughout therapy.
```