Xaprost Eye Drop, Travoprost
- 1. Introduction to Xaprost Eye Drop (Travoprost)
- 2. Medical Uses of Xaprost Eye Drop
- 3. Travoprost Mechanism of Action: How Xaprost (Travoprost) Works
- 4. Composition and Formulation
- Active Ingredient: Travoprost 0.004%
- Inactive Ingredients and Preservatives (e.g., benzalkonium chloride or preservative-free formulations)
- Physical Properties: pH, Appearance, and Viscosity
- Travoprost vs Bimatoprost
- Travoprost vs Latisse
- Tafluprost vs Travoprost
- Travoprost vs Timolol
- Travoprost vs Travatan Z
- Travoprost vs Lumigan
- Travoprost vs Latanoprost
- Travoprost Alternative
- 5. Travoprost Dosage and Administration Guidelines
- 6. Travoprost Eye Drop Side Effects
- Overview of Possible Side Effects
- Short-Term vs. Long-Term Effects
- Frequency and Severity Classification
- 6.1 Common Side Effects
- Eye Redness (Hyperemia)
- Ocular Irritation or Burning Sensation
- Eyelash Changes (Growth, Thickening, Darkening)
- Increased Pigmentation of the Iris
- Dry Eye Symptoms
- 6.2 Serious and Less Common Adverse Reactions
- Eye Infections or Conjunctivitis
- Visual Disturbances
- Eyelid Discoloration
- Macular Edema
- Uveitis or Iritis
- 7. Travoprost Drug Interactions and Compatibility
- 8. Travoprost Warnings and Contraindications
- 9. Guidelines for Careful Administration
- 10. Important Precautions Before and During Use
- 11. Special Considerations in Specific Populations
- 11.1 Administration to Elderly Patients
- Adjustments in Dosage or Monitoring Frequency
- Age-Related Sensitivity to Side Effects
- 11.2 Administration to Pregnant Women and Nursing Mothers
- Travoprost Nursing Considerations
- Travoprost Nursing Implications
- Travoprost Nursing Interventions
- 11.3 Administration to Pediatric Patients
- Safety and Efficacy in Children and Adolescents
- Clinical Guidelines for Use in Congenital Glaucoma
- 12. Overdose and Emergency Management
- 13. Storage and Shelf Life
- 14. Handling Precautions and Disposal
1. Introduction to Xaprost Eye Drop (Travoprost)
What is Xaprost Eye Drop?
The Xaprost Eye Drop is a type of eye treatment that includes travoprost as the active ingredient, which is a synthetic version of prostaglandin F2 alpha that helps lower high pressure inside the eye (known as intraocular pressure or IOP). This medication is primarily used in patients with open-angle glaucoma or ocular hypertension to reduce their eye pressure levels and prevent nerve damage caused by increased intraocular pressure (IOP) by improving the drainage of fluid from the eye. This eye drop is designed to be used on the eyes once a day. It is commonly included in long-term treatment strategies that require steady control of intraocular pressure (IOP).

Overview of Travoprost as a Prostaglandin Analog
Travoprost is classified as a type of medication known as prostaglandin analogues, which imitate the function of prostaglandins found naturally in the body by attaching to receptors in the eye, specifically those known as prostaglandin FP receptors. The result of this interaction is a modification in the pathway that enhances fluid drainage within the eye.
Essential characteristics of travoprost:
- Highly particular, about FP receptors.
- The extended period of effectiveness allows the medication to be taken once daily.
- Proven effectiveness in lowering pressure in groups of patients.
- Appropriate for use on its own or in combination with beta blockers.
Travoprost is commonly used in ophthalmology as a treatment for ocular hypertension and glaucoma due to its strong effectiveness and well-received tolerability.
Regulatory Status and Availability in Different Countries
Travoprost-based items, such as Xaprost Eye Drops, have gained acceptance. They are authorized under different brand names worldwide. The medication is only approved by the FDA for prescription in the United States.
- The European Union has given its approval through the European Medicines Agency (EMA) for use in all member countries.
- Japan has given its approval to brand designations as, per the PMDA guidelines.
- Australia and New Zealand are included in the prescription schedules, with approval from the TGA and Medsafe.
- In India and Southeast Asia, Xaprost and other generics are readily accessible, with no prescription requirements. Regional variations in labeling may differ based on guidelines from the drug authority, particularly in factors such as the preservatives used in products or the concentration and size of the bottles involved.
Travoprost Drug Classification
Travoprost falls into the chemical categories listed below:
- S01EE04 is the AT Code for Prostaglandin analogues used in ophthalmology.
- Class of Medication: This drug belongs to a category of medications known as prostaglandin analogs and is classified accordingly.
- Mechanism-Based category, for enhancing uveoscleral outflow., It's commonly used as a choice for treating glaucoma due to its ability to effectively manage long-term eye pressure while causing minimal side effects in the body.
2. Medical Uses of Xaprost Eye Drop
Approved Uses
Reduction of Elevated Intraocular Pressure (IOP)
- It has been demonstrated to reduce pressure (IOP) by 25–32 percent compared to the level.
- The maximum impact is typically noticed around 12 hours after taking the medication.
- Ensures management of pressure levels through a daily dose.
This profile of how the medication works reinforces its importance in managing eye pressure.
Treatment of Open-Angle Glaucoma
Patients with open-angle glaucoma (POAG) often rely on Xaprost as a treatment option. Because it helps improve outflow, it works against the impaired drainage through the meshwork, a common feature of this long-term optic nerve condition. Ideal for individuals in the phases and mid-stages of open-angle glaucoma. It can be recommended for use, either alone or in conjunction with beta blockers or carbonic anhydrase inhibitors. Extended usage of the product has been observed to slow down the progression of visual field degradation over time. Its ability to be well-tolerated by the eyes and its effectiveness make it a good option for long-term use in treating glaucoma.
Ocular Hypertension Management
Treatment of hypertension, with Xaprost is sanctioned when no signs of glaucomatous damage are present in patients Individuals with high intraocular pressure (IOP) but with normal visual fields and optic nerves are still susceptible, to developing glaucoma in the future.
- In individuals, at risk (such as those, with corneas or a family history of the condition) using preventive measures is recommended.
- It lowers the chance of developing nerve damage.
- Before opting for procedures or laser treatments, it is advisable to consider invasive treatment options.
Off-Label Uses
Use in Normal-Tension Glaucoma
Travoprost although not formally sanctioned for normal tension glaucoma (NTG) has displayed potential, in treating individuals, within this group who experience harm with seemingly normal IOP levels.
- Assists in lowering eye pressure levels beyond the thresholds.
- In individuals with tension glaucoma, it may help to stabilize vision in patients with dysregulation.
- Make sure to select patients and monitor the optic nerve regularly.
Adjunctive Therapy with Other Intraocular Pressure-Lowering Agents
Sometimes Xaprost is used in combination with medications, but when used alone, it doesn't reach the desired blood pressure levels.
- When used with timolol, dorzolamide, or brimonidine, it improves effectiveness.
- Using fixed-dose combination medications helps reduce the need for medications and simplifies treatment.
- To prevent any impact, it is necessary to administer the medication at intervals.
This method of layer application proves beneficial in challenging cases of refractory glaucoma.
Cosmetic Use for Eyelash Growth (controversial and unapproved)
People have started using containing solutions in medical ways to enhance their eyelashes for cosmetic purposes after seeing the positive results of similar prostaglandin analogues, in dermatology, for aesthetics.
- There have been reports of eyelashes appearing longer and thicker, with a hue. The authorities have not approved this study.
- Using these products may lead to some risks, such as darkening around the eyes ( hyperpigmentation), redness in the eye area (conjunctival hyperemia), and alterations in the color of the iris.
It's essential to proceed when using products because of safety worries and ethical factors involved in recommending them.
3. Travoprost Mechanism of Action: How Xaprost (Travoprost) Works
Role of Prostaglandin F2α Analog in Intraocular Pressure Reduction Save Cancel
Enhancement of Uveoscleral Outflow
Travoprost works differently from medications that reduce fluid production in the eye by enhancing the outflow pathway instead of suppressing aqueous humor production like other drugs do. It mainly targets the muscle and supraciliary space to increase this route and is known to be more effective than other methods commonly used for this purpose due to its stimulation by prostaglandin analogues.
- Improves the flow of eye fluid outside the mesh structure.
- Enhances the permeability of the matrix in the muscle.
- Encourages maintaining pressure levels with absorption into the body.
By utilizing this natural drainage process in the body system, Xaprost effectively manages pressure (IOP) and maintains the functionality of traditional pathways for outflow.
Onset and Duration of Action
Travoprost shows a profile in terms of its pharmacokinetics and pharmacodynamics.
- The maximum impact is usually achieved around the 12-hour mark.
- The effects last, for 24 hours providing a continuous reduction, in eye pressure.
- These features make it easy to take the medication every day.
It is usually recommended to take it in the evening to match its best performance with the increase in eye pressure that often happens in the early morning, which is a critical time for worsening glaucoma symptoms.
Comparison with Other Prostaglandin Analogues
Travoprost is part of a group that also includes latanoprost, bimatoprost, and tafluprost. Though they all work by activating the FP receptor, each has pharmacological and formulation variances.
- Travoprost is recognized for its ability to lower pressure in various ethnic populations and is also offered in preservative-free formulations.
- Bimatoprost has an attachment, though it might lead to cosmetic issues, like pigmentation, around the eyes.
- Tafluprost is a choice for people with eyes because it comes in a preservative-free form.
Travoprost's rounded effectiveness and gentle impact on the eyes, combined with its various formulation choices, make it a top contender in the treatment of glaucoma using prostaglandins.
4. Composition and Formulation
Active Ingredient: Travoprost 0.004%
The Xaprost Eye Drop has travoprost as its ingredient with a strength of 0,004%, which is equal to 40 micrograms per milliliter (40 micrograms/mL). This synthetic prostaglandin F₂α is available in an eye solution for use on the surface of the eye. The therapeutic benefit is achieved by activating the FP receptor. The medication is provided in containers that can be used multiple times, with the option of including preservatives or not. The medication shows a preference for receptors and maintains a lasting decrease in pressure inside the eye. The focus guarantees the results, with impact, on the overall system.
Inactive Ingredients and Preservatives (e.g., benzalkonium chloride or preservative-free formulations)
The mixture consists of additives to uphold the stability of the solution and ensure it is isotonic and sterile.
- Benzalkonium chloride (BAk) is often utilized as a preservative; however, it may cause irritation on the surface of the eye for individuals.
- For those sensitive to BAK, preservative-free Zia preserved options are available.
- The list of ingredients includes mannitol, boric acid, sodium chloride, tromethamine, and purified water.
These components help maintain the product's chemical stability while also ensuring it remains gentle on the eyes.
Physical Properties: pH, Appearance, and Viscosity
Travoprost eye drops are known for their characteristics.
- The pH range is around 5.5 - 6.5. It is slightly acidic to align with the pH of the eyes.
- The solution appears transparent with a hint of color.
- The liquid is easy to absorb and disrupts vision after applying it.
These features are meticulously fine-tuned to improve the diffusion of medication while keeping the patient's discomfort to a minimum.
Travoprost vs Bimatoprost
Both agents are classified under the prostaglandin analog category.
- Travoprost is an FP receptor agonist, with a safety record and a reduced occurrence of hyperemia.
- Bimatoprost is classified as a prostamide. It is known to be more effective in certain situations. However, it is likely to cause side effects such as increased eyelash growth and changes in pigmentation. Using bimatoprost might lead to a decrease in intraocular pressure but may come with more noticeable changes in appearance.
Travoprost vs Latisse
Latisse, which contains bimatoprost 0,03%, has been approved by the FDA for enhancing eyelashes.
- Travoprost is not authorized to promote eyelash growth, and using it for purposes not specified on the label can pose risks.
- Latisse is designed to be applied to the skin along the lash line. It is not meant for use inside the eye.
Although they share similarities at the level their compositions differ in terms of recommended uses and safety characteristics.
Tafluprost vs Travoprost
- Tafluprost belongs to a class of prostaglandin analogs.
- Tafluprost is a choice for those with eyes or allergies, as it is preservative-free.
Travoprost is becoming increasingly accessible to the public. It is notably found in fixed-dose combinations. Tafluprost might provide comfort compared to others; however, its real-world effectiveness is typically similar.
Travoprost vs Timolol
- Timolol is a type of beta blocker that is not explicitly targeted. It is utilized to reduce pressure (IOP).
- Travoprost enhances the drainage of eye fluid. It is taken once a day
Timolol helps decrease the production of fluid in the eye and is usually taken twice a day. Travoprost is a choice for patients with asthma or bradycardia since it doesn't have beta-adrenergic effects.
Travoprost vs Travatan Z
Travatan Z is a version of travoprost produced by a known brand. Generic travoprost, known as Xaprost, includes benzalkonium chloride. Travatan Z utilizes sofzia as a system. Travatan Z might be better received by individuals dealing with standing eye or issues with the surface of the eyes.
Travoprost vs Lumigan
The medication Lumigan includes a compound called bimatoprost that varies in both structure and function.
- Travoprost exhibits activity specific to the FP receptor, providing a combination of results and safety.
- Lumigan has a prostamide effect, which is linked to an increase in pigmentation side effects.
The choice of medication is based on how the patient reacts to it their ability to tolerate side effects and the desired treatment outcomes.
Travoprost vs Latanoprost
Latanoprost is commonly chosen as the prostaglandin analog because of its proven effectiveness.
- Latanoprost is considered stable and generally well tolerated; however, it may have a potency compared to other options.
- Travoprost possibly decreases intraocular pressure among African American individuals. It might provide benefits in groups or for complex cases.
Travoprost Alternative
Other options besides travoprost may involve agents.
- Other medications in the class, as prostaglandin analogues, include latanoprost, tafluprost, and bimatoprost.
- Non prostaglandin medications include timolol, brimonidine and dorazolamide.
- Enhancing control of pressure through the use of combination eye drops such as travoprost, timolol, and bimatoprost.
Factors like the patient's needs and health conditions should influence the selection of an option based on effectiveness and possible side effects for their eyes' well-being.
5. Travoprost Dosage and Administration Guidelines
Recommended Dosage for Adults
For grown-up patients, the usual advised amount of Xaprost Eye Drop ( 0.004%) is one drop in the affected eye(s) daily during the evening, as this matches the natural rhythm of eye pressure, which usually peaks in the early hours of the morning.
- Please do not use than one drop in each eye on a daily basis.
- Frequent use could reduce the effectiveness of lowering eye pressure.
Consistently following the prescribed dosage is crucial to ensure the treatment remains effective.

Frequency and Timing of Application
Travoprost is designed to be used daily because it stays effective for a time in the body's system.
- Taking it at the time every day in the evening helps maintain stable control of eye pressure.
- Taking the medication in the evening helps to lower eye pressure during the night.
- Repeated administration within a day may affect the sensitivity of receptors.
Clinical findings indicate that not following the recommended dosage schedule could weaken the medication's effectiveness in the long run.
Instructions for Proper Use and Eye Drop Instillation
The instillation technique is crucial for ensuring the delivery of medication and minimizing the chances of contamination or absorption into the body.
- Remember to wash your hands before touching the bottle.
- Gently tilt your head backward.
- Pull down the eyelid to create a small pouch in the conjunctiva.
- Add a drop into the pocket without letting the dropper tip touch the eye or its surroundings.
- Gently close your eyes.
- Gently press the corner of the eye for 1 to 2 minutes to reduce the amount absorbed into your system.
Remember to remove any liquid and put the cap back on the bottle. Do not wear contact lenses while using eye drops containing preservatives, like benzalkonium chloride.
Missed Dose Instructions
If you forget to take a dose of medication, be sure to take it on the day you remember.
- If its almost time, for the dose as, per schedule skip the one you missed.
- Please do not increase the dosage to make up for it.
- Excessive consumption could result in changes, in eye pressure or irritation, in the eyes.
Duration of Treatment and Long-Term Use Considerations
Travoprost is meant for patients who need to reduce pressure inside the eye over the long term. Treatment duration is usually open-ended and adjusted based on how the disease progresses and how the patient responds. Regularly checking the pressure (IOP) condition of the nerve and visual field is crucial.
Extended use might result in eye-related alterations, like increased eyelash length or changes in the color of the iris or pigmentation of the skin around the eyelids. Therapy needs to be reviewed to make sure it's still helping and that patients are following through with it. If the pressure control is not sufficient or if any reactions are observed, other treatment options may be taken into account, depending on the doctor's assessment.
6. Travoprost Eye Drop Side Effects
Overview of Possible Side Effects
While Travoprost is usually well-received by people's eyes and surrounding areas without causing issues or discomfort overall, it might lead to various eye-related problems, such as temporary irritations or even long-lasting alterations in the color of eye tissues due to pigmentation changes. Most of the impacts are limited to areas around the eyes; however, in rare cases, some systemic effects could arise from minimal absorption into the body overall.
The frequency and seriousness of reactions may differ depending on tolerance levels, how long the product is used, and its composition (with or without preservatives). Some impacts may improve with use, while others might require stopping or replacing. It is advisable to monitor patients to assess their ability to tolerate treatment and identify any complications at an early stage.

Short-Term vs. Long-Term Effects
The appearance and duration of side effects typically adhere to a schedule.
- After starting the treatment, you may experience short-term effects, like burning sensations, stinging, and redness. These symptoms usually appear within hours to days after beginning the medication.
- Over time, the pigmentation and features of lashes evolve slowly, taking weeks to months to develop fully.
Although most side effects may be minor and harmless, in appearance, their influence over following treatment guidelines should not be disregarded.
Frequency and Severity Classification
Different side effects are categorized based on how they occur and how severe they are in a clinical setting.
- Frequent (>10%); Red. Alterations in eyelashes. Some typical side effects may include irritation, itching, and pigmentation on the eyelids.
- Visual disturbances and eye inflammation, such as uveitis and conjunctivitis, are considered side effects (occurring in 0.1% to 1% of cases).
- In cases (< 0.1%), Macular edema or Periorbital fat loss may result in varying levels of discomfort or potentially serious vision-related issues for certain people who are susceptible to them.
6.1 Common Side Effects
Eye Redness (Hyperemia)
Concomitant redness of the eyes is commonly noted as an effect of using travoprost, attributed to the widening of blood vessels on the surface of the eye.
- Typically, it is mild and resolves on its own.
- The effects may decrease over time with usage.
- One of the reasons why some patients stop therapy early is due to their sensitivity.
Ocular Irritation or Burning Sensation
Immediately after applying some people may feel a stinging or burning sensation.
- It is more typical to see familiar phrases maintained.
- Switching to preservative options might help reduce the issue.
Eyelash Changes (Growth, Thickening, Darkening)
Travoprost encourages the growth phase of eyelash follicles known as anagen.
- Achieving fuller eyelashes.
- The pigmentation has intensified.
- Thicker eyelashes
Asymmetrical eyelash growth is often seen as an issue, but it can also be unattractive or make people wonder if there's been some misuse involved.
Increased Pigmentation of the Iris
The production of melanin in melanocytes may increase in the iris of people with colored irises. Over time, there is a lasting transformation. In cases where one eye is treated, there is no evidence of any effects on clarity or functionality. Patients ought to be advised regarding the possibility of darkening of the iris.
Dry Eye Symptoms
In cases, prostaglandin analogues can worsen dry eye symptoms for some people.
- Factors that play a role include preservatives and a rise in film evaporation.
- Common symptoms of this condition may include a sensation of grittiness in the eye and increased sensitivity to light.
- It might be necessary to consider using tears as a treatment option or to reassess the current therapy.

6.2 Serious and Less Common Adverse Reactions
Eye Infections or Conjunctivitis
Travoprost may occasionally increase the risk of eye surface infections or allergic conjunctivitis in users.
- Symptoms may include discharge from the eyes and swelling or itching of the conjunctivae.
- Distinguishing between an allergic cause is necessary for the diagnosis.
- In cases of infection, it may be necessary to discontinue the treatment and consider therapy as a potential course of action.
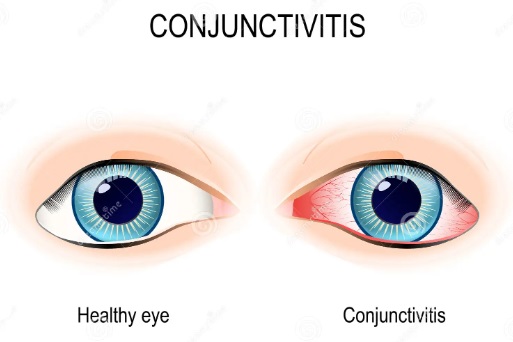
Visual Disturbances
If the drops are not applied properly, vision may become blurry, and clarity may fluctuate. This is typically temporary, but in cases where the issue persists, it's crucial to investigate any structural issues.

Eyelid Discoloration
Prolonged use may lead to darkening of the skin around the eyes, known as hyperpigmentation, in the eyelid or periorbital area. This is connected to the buildup of melanin in the layers of the skin. The treatment typically targets one eyelid, the one.
Macular Edema
Rarely occurring but significant complication, Cystoid macular edema (CME) is of concern in individuals who have undergone cataract surgery. Blurred or distorted central vision can be noticed as a symptom. It might be necessary to have an eye examination and consider stopping the treatment. Patients who have had issues with their region in the past need to be observed.
Uveitis or Iritis
Susceptible individuals have been known to experience inflammation within the eye, such as uveitis or iritis. The individual is experiencing pain in the eye area, along with sensitivity to light (photophobia), decreased vision quality, and redness. Upon confirmation, it is recommended to stop the medication and start inflammatory treatment.
7. Travoprost Drug Interactions and Compatibility
Interaction with Other Ophthalmic Solutions
- Remember to apply types of eye drops with a gap of 5 minutes between each application.
- Apply ointments or gels as a step to prevent any disruption in the absorption of the solution.
- Try not to put eye drops in your eye at the same time.
When one medication alone is not enough, it is often considered acceptable to use it with beta-blockers (such as timolol), alpha agonists (like brimonidine), or carbonic anhydrase inhibitors (e.g., dorzolamide).
Systemic Interactions (Minimal Absorption, Low Systemic Risk)
Travoprost is bioavailable because it is administered locally and metabolizes quickly in the eye tissues.This lowers the chances of any interactions affecting pharmacokinetics or pharmacodynamics significantly.
- The acid levels in the plasma are usually too low to be detected.
- No noteworthy interactions have been documented with medications used to treat blood pressure or blood thinners or drugs for diabetes.
- Serious side effects, like bronchospasm and bradycardia are very uncommon, in the system.
Travoprost does not affect the liver's cytochrome P450 enzymes and is not believed to change how drugs are cleared from the body systemically.
Precaution with Concurrent Use of Prostaglandin Analogues
The simultaneous use of multiple prostaglandin analogues or derivatives is contraindicated or discouraged, as it may paradoxically elevate intraocular pressure or attenuate therapeutic response.
- Risk of receptor desensitization or competitive inhibition.
- Potential for increased ocular side effects such as hyperemia, irritation, or pigmentation.
- No evidence of additive benefit in IOP reduction when combined with other prostaglandins.
- If switching from one prostaglandin analog to another, a washout period may be recommended based on the clinician's discretion.
Therapy duplication should be avoided unless under strict ophthalmologic supervision.
8. Travoprost Warnings and Contraindications
Contraindicated in Hypersensitivity to Travoprost or Any Component
Individuals who are known to be sensitive to Travoprost or any of its components should avoid using the medication due to reactions that could lead to eye irritation and other symptoms, such as redness and swelling.
- If you notice any signs of hypersensitivity, stop using it. When pondering treatments, for reducing pressure inside the eye it's worth exploring options.
- Before starting treatment, it's important to assess patients who have a history of reactions to drugs.
Travoprost should be used with caution in individuals who have sensitivities, as it could potentially cause increased immune-related eye issues.
Precaution in Patients with Active Ocular Infections
- Wait to start until the infection has completely cleared.
- In instances of recurring eye conditions, it's important to weigh the risks against the benefits carefully.
- If the treatment cannot be delayed because of an increase in pressure, it is essential to monitor the situation closely.
Topical medications, like prostaglandin analogues, could affect the permeability of the blood barrier and might worsen inflammation inside the eye.
Risk of Changes in Eyelash and Iris Pigmentation
Continuous and potentially lasting alterations in eye coloration have been linked to the utilization of travoprost over time, especially when used on one eye for an extended period.
- Enhanced eyelash appearance results from boosted follicle stimulation, leading to thicker lashes and a hue.
- The color of the iris can gradually darken over time in people, with irises that have a mix of colors, like blue brown hues.
- Hyperpigmentation may cause changes in the color of the skin around the eyes. These changes could potentially be reversed.
- The alterations, in skin color are usually harmless and do not cause any discomfort; however they can be a source of worry for some individuals.
Patients should be made aware of these changes before starting treatment, especially in cases where only one side of the body is being treated, as this can lead to noticeable differences over time.
9. Guidelines for Careful Administration
Proper Hygiene and Sterile Application Technique
To reduce the chances of eye irritation or additional infections occurring, it is crucial to practice when applying the product.
Before using it each time:
- Remember to wash your hands with soap and water.
- Make sure there are no makeup products or dirt residue on your face and eyelids.
- Be sure to dab the area around your eye with a dry towel.
The medication should be applied directly into the space around the eyes without touching the eyelashes, lids, or any nonsterile surface.
Avoiding Contamination of the Dropper Tip
The effectiveness of the solution greatly relies on keeping the dropper tip clean during its use.
- Avoid letting the bottles' tips come into contact with your eyes, fingers, or any surface.
- Always remember to seal the bottle as soon as you're done using it.
- Make sure to keep the bottle standing up in a spot that's clean and dry.
Cross-infection may cause eye infections such as keratitis and conjunctivitis in people with weakened immune systems.
Use with Contact Lenses: Timing and Material Considerations
Travoprost formulations frequently include preservatives such as benzalkonium chloride, which has the potential to attach to and break down contact lenses.
- Remember to take out your contact lenses before putting in eye drops.
- Remember to wait for 15 minutes before putting your contact lenses back in.
- Opt, for silicone hydrogel contact lenses or daily disposables to minimize the build up of preservatives.
Patients with eyes or who need to use lenses frequently may find preservative-free travoprost versions more appropriate.
Precaution in Unilateral Use (Cosmetic Asymmetry)
When one eye is treated with travoprost, the appearance of changes may become more noticeable over time. The iris of the eye that received treatment showed more color than the other eye.
- One side of the eyelashes appears to have improved in terms of length and thickness.
- The darkening of the skin around the eyes or the reduction of fat in a region, after treatment.
Making these alterations usually doesn't cause any harm, but they could end up being permanent in some cases, so it's essential to talk to patients before starting any treatment to manage their expectations and ensure they follow through diligently.
10. Important Precautions Before and During Use
Regular Monitoring of Intraocular Pressure
Regularly checking the pressure inside the eye ( pressure or IOP) is crucial for making sure that treatments are working well and for spotting any issues, like not responding well to treatment or the condition getting worse over time. Even though Travoprost is reliable in lowering IOP in a person's eye, pressure levels may vary, and so it's important to keep an eye on it in a way that suits each individual specifically.
- It is essential to determine the IOP readings before starting.
- It's advisable to reassess the situation 2 to 4 weeks after starting the treatment.
- Regular monitoring every 3 to 6 months is crucial, for individuals, with glaucoma or ocular hypertension.
Changes, to the treatment plan might be necessary if there are variations in pressure levels or if control is not adequate—especially when dealing with damage, from glaucoma.
Risk of Iris and Eyelid Pigmentation with Prolonged Use
Continuous use of travoprost may result in darkening of eye structures caused by the stimulation of melanin production.
- People with hazel eyes might notice their irises darkening slowly over several months of treatment.
- This change in iris pigmentation is quite common among them.
- Usually, there's a darkening of the skin on the eyelids and around the eyes that can go away once you stop whatever is causing it.
- In cases where one side is treated differently from the other in a procedure, like surgery or injections to enhance appearance,
- This requires discussion with patients who're particularly concerned about aesthetics.
These changes in pigmentation are harmless. They do not impact vision but may affect how satisfied one feels with one's treatment's appearance.
Importance of Adherence to Dosage Regimen
Adherance, to the recommended dosages is vital for getting the results, in treating eye pressure effectively.
- Travoprost is meant to be used once daily; using it more often could actually make it less effective.
- Missing doses may cause an increase in pressure within the eye. Raise the chances of causing harm to the optic nerve.
- Encouraging patients to stick to a dosing schedule, such, as taking medication every night helps improve consistency and leads to clinical outcomes.
Ensuring that individuals receive education on following guidelines and proper execution enhances sustained management and reduces the likelihood of therapy shortcomings.
11. Special Considerations in Specific Populations
11.1 Administration to Elderly Patients
Adjustments in Dosage or Monitoring Frequency
Older patients play a role in the treatment of glaucoma and eye pressure issues. An overall dosage adjustment based solely on age is usually unnecessary; however, it's recommended to implement personalized monitoring approaches because of the occurrence of health conditions and multiple medication use among this group.
- Begin with the dosage unless there are any reasons not to use it due to other eye-related issues.
- We should check the eye pressure often in patients with optic nerve damage.
- Assess the kidney and liver function in cases where there might be issues with absorption.
Senior individuals might need assistance with applying techniques because of decreased hand coordination or eyesight issues.

Age-Related Sensitivity to Side Effects
Elderly individuals may experience increased vulnerability to eye and eyelid issues linked to the use of treatment.
- The higher chance of experiencing eye syndrome or irritation on the surface has increased.
- Inflammatory reactions, like conjunctivitis or uveitis, may take longer to resolve.
- The combined impact of changes such as darkening of the eyelids could appear noticeable because of the thinner skin around the eyes.
It's important to have eye checkups as you get older to ensure that the benefits of treatment outweigh the risks to your eyes' health.
11.2 Administration to Pregnant Women and Nursing Mothers
Travoprost Nursing Considerations
Travoprost is classified under FDA Pregnancy Category C. While animal studies have shown adverse effects on the fetus, adequate and well-controlled human studies are lacking.
- Use only if the potential benefit justifies the potential risk to the fetus.
- Avoid during early gestation unless essential
- Consider alternative agents with a more favorable pregnancy safety profile
The possibility of systemic absorption, though minimal, warrants caution in this sensitive population.
Travoprost Nursing Implications
The uncertainty surrounding the excretion of travoprost into breast milk among breastfeeding mothers is not well defined due to a lack of human research data on the matter; given its lipid-soluble nature, the likelihood of transfer cannot be ruled out.
- Please provide the text you would like me to assist in paraphrasing, or ask any questions you may have.
- When possible, it is advisable to choose agents with known safety records for lactation.
- If you must undergo treatment that cannot be avoided and involves applying a substance or medicine to your body that may affect breastfeeding infants in any way or form through exposure to the substance or medicine present in breast milk after application is complete.
It is advisable to express breast milk and dispose of it post-application to reduce the possibility of infant exposure. Before starting, it's essential to educate patients who are breastfeeding about the consequences.
Travoprost Nursing Interventions
When caring for patients using travoprost, nurses administer treatments.
- Educating individuals on the method of occlusion to minimize the absorption into the bloodstream.
- Coordinating eye care appointments alongside appointments with obstetricians or pediatricians.
- After the exposure, make sure to note down any symptoms or negative reactions the baby may experience.
Ensuring the safety of mothers and newborns requires a team effort involving experts from various fields working collaboratively.
11.3 Administration to Pediatric Patients
Safety and Efficacy in Children and Adolescents
Travoprost is not widely approved for children because there isn't long-term safety information about its use in pediatric populations, yet it may be used off-label under the close monitoring of a specialist for treating pediatric glaucoma.
- Clinical studies involving children aged two and above have shown effectiveness; however, redness is frequently observed.
- Healthcare providers should keep an eye on monitoring, for effects, in children compared to adults.
When considering starting treatment, it's important to balance the advantages with the risks of lasting effects on skin color or eye health. Parents' commitment and guidance are essential for the care of children.

Clinical Guidelines for Use in Congenital Glaucoma
In situations involving juvenile glaucoma cases when surgical treatments are not immediately available or suitable, travoprost can be incorporated as a component of a treatment strategy.
- Start with the amount and monitor closely through regular eye check-ups.
- Keep an eye out for eyelashes or any changes, in pigmentation that could impact the self esteem of older children.
- When needed and if necessary, for lowering eye pressure levels consider using in conjunction, with medications.
- Avoid using two prostaglandin analog treatments at the same time.
Expert guidance from pediatric eye doctors is crucial to ensuring the successful incorporation of travoprost into the treatment plan for glaucoma.
12. Overdose and Emergency Management
Symptoms of Accidental Overuse
Travoprost has a safety margin when applied to the eyes; however, using it too much accidentally can lead to reactions either in the treated area or throughout the body.
- The eyes appear red and irritated more than usual.
- The swelling or irritation of the eyelids is noticeable.
- Heightened stimulation of prostaglandin-induced impacts, like eyelash growth or darkening around the eyes, is also noticeable.
Using the medication often than directed could actually decrease its effectiveness in lowering eye pressure because the receptors may become less responsive over time.

Systemic Toxicity Risk and Management
Does systemic toxicity occur from applying travoprost because it does not reach significant levels in the bloodstream after absorption through the eyes? However, it is essential to be cautious when using doses or when treating patients with less efficient clearance mechanisms. Some individuals might experience low blood pressure, feelings of lightheadedness, or fatigue. Symptoms can be managed effectively without the need for an antidote.
Keep an eye on the heart and breathing health of at-risk individuals, such as children and older adults. In case of a reaction, the key approach is providing supportive care by monitoring fluid balance and vital signs closely.
Recommended Actions in Case of Oral Ingestion
If someone accidentally swallows it – children – it's best to seek help right away when dealing with Travoprost ingestion orally is generally low, in toxicity when taken in small doses but can still be risky if ingested in large quantities.
- Make sure to rinse your mouth with water to minimize leftover absorption.
- Only induce vomiting if a healthcare provider specifically advises you to do.
- If there has been an recent ingestion consider giving charcoal under the direction of a medical professional.
Measures to assist should be taken according to the patient's symptoms. Reaching out to poison control services and emergency medical help is advised for any serious exposures, especially in children and patients with compromised health conditions.
13. Storage and Shelf Life
Recommended Storage Conditions (Temperature, Light, Moisture)
Ensuring the eye drops remain stable and sterile requires following the recommended storage instructions diligently to maintain their effectiveness and prevent contamination risks.
- Please store in a place with a room temperature between 2 °C and 25°C (36°F and 77°F).
- Remember to shield the item from sunlight or intense heat.
- Remember to safeguard the contents from moisture and humidity by securely sealing the bottle when not in use.
- Please do not allow the solution to freeze because it could make it ineffective or unstable.
Storing it in a tidy and dry medicine cabinet or a refrigerator drawer is recommended for preserving the item's quality and effectiveness, which is beneficial in regions with warmer climates.

Shelf Life After Opening
After you open the bottle and store it properly the sterility and preservative system begin to deteriorate over time.
- After you open it, the typical shelf life is about four weeks to a month.
- It is advisable to mark the date of use, on the bottle.
- If you notice any differences in color or texture, or if it looks cloudy in any way, it's best to throw it out.
Extended usage, beyond the timeframe, raises the chances of contamination occurrence that could result in severe eye infections.
Discarding Unused or Expired Medication
Appropriately getting rid of expired or unused travoprost is crucial for maintaining safety and protecting the environment.
- Please refrain from disposing of the solution in the toilet or pouring it down drains.
- Remember to bring any medication you didn't use to a pharmacy that accepts returns.
- Make sure to dispose of it according to your local regulations for medical waste.
Remember to store this product in a place where children and pets cannot access it to avoid any contact. In healthcare facilities, it's essential to follow the guidelines for disposing of medications to meet regulations and prevent waste from harming the environment.
14. Handling Precautions and Disposal
Safe Handling for Patients and Caregivers
To ensure that Travoprost is administered safely, it's important to use it and handle it with care. Contamination or mishandling can put both safety and effectiveness at risk.
- Remember to wash your hands both before and after touching the bottle.
- When using the dropper tip, avoid touching it with your fingers or the surface of your eye or skin.
- Please do not give the medication to anyone, even if they are showing symptoms.
- If the remedy touches any surfaces by accident, make sure to clean that area thoroughly.
Care providers should be extra cautious when giving medication to others to ensure cleanliness and avoid mixing up medications.
Proper Disposal Methods for Used Containers
Once you finish the course, the term expires, and the use period is up.
- Make sure to dispose of any used containers properly to prevent them from being mistakenly reused or causing exposure risks.
- Remember to put the cap on before throwing it away to avoid any leaks.
- Please dispose of the container in a designated medical waste bin.
- Take it back, to a pharmacy, for disposal.
- Please avoid crushing or burning plastic containers, as it could result in the release of substances.
It's best to avoid tossing pharmaceuticals in the household trash if there are disposal facilities for them, particularly in institutional or clinical environments.
Environmental Safety Considerations
Pharmaceutical remnants, such as eye medications, can lead to pollution if not disposed of correctly. Taking care of substances like Travoprost is crucial for reducing their footprint.
- It's best to refrain from disposing of medication in toilets or sinks because wastewater systems may not completely remove substances.
- Engage in community programs for the disposal of medications to promote friendly practices.
- Inform patients, about the impact, on the environment when waste is not disposed of properly.
- Focus on how this can affect ecosystems.
Maintaining both personal safety and environmental sustainability standards effectively and responsibly requires following handling and disposal procedures.
Xaprost Eye Drop, Travoprost FAQ
- Does Travoprost Need To Be Refrigerated
- How Does Travoprost Work
- How Long Is Travoprost Good For After Opening
- How Travoprost Works
- Is Travatan And Travoprost The Same
- Is Travoprost Preservative Free
- Is Travoprost The Generic For Travatan Z
- Is Travoprost The Same As Travatan Z
- What Is The Difference Between Latanoprost And Travoprost
- What Is The Difference Between Travatan And Travoprost
- What Is The Difference Between Travatan Z And Travoprost
- What Is Travoprost
- What Is Travoprost Eye Drops For
- What Is Travoprost Ophthalmic Solution Used For
- What Is Travoprost Used For
- Which Condition Is Indicated For A Patient Presenting A Prescription For Travoprost To Be Filled?
Does Travoprost Need To Be Refrigerated
Nope! Travoprost doesn't need to be kept in the fridge. Store it at room temperature between 15°C and 25°C (59°F–77°F).
How Does Travoprost Work
Travoprost is a type of prostaglandin analogue that reduces intraocular pressure by enhancing fluid drainage from the eye.
How Long Is Travoprost Good For After Opening
After opening, Travoprost is generally effective for up to 6 weeks. Be sure to follow the guidelines provided with the product.
How Travoprost Works
Travoprost reduces eye pressure by improving the flow of liquid from the eye.
Is Travatan And Travoprost The Same
Travoprost serves as the active component in Travatan; however, Travatan is a marketed formulation.
Is Travoprost Preservative Free
Travoprost formulations typically include preservatives, such as benzalkonium chloride, in some cases. There are also preservative-free options available, although not always.
Is Travoprost The Generic For Travatan Z
Certainly! Travoprost is the generic version of Travatan Z.
Is Travoprost The Same As Travatan Z
Travatan Z and its generic counterpart both contain Travoprost as the active ingredient. However, Travatan Z distinguishes itself by employing benzalkonium chloride as its preservative.
What Is The Difference Between Latanoprost And Travoprost
Both Travoprost and Bimatoprost are types of prostaglandin analogues that are prescribed to reduce pressure in the eyes; however, Travoprost is often preferred for patients with pigmented irises due to its higher efficacy levels and greater potency compared to Bimatoprost.
What Is The Difference Between Travatan And Travoprost
Travatan and Travoprost are essentially the same, differing only in their brand names, as they share the same active ingredient.
What Is The Difference Between Travatan Z And Travoprost
Travatan Z contains Travoprost. Utilizes a preservative called Sofzia instead of benzalkonium chloride in order to minimize eye irritation.
What Is Travoprost
Travoprost, a type of prostaglandin analog, is utilized to reduce pressure within the eye in conditions such as glaucoma or ocular hypertension.
What Is Travoprost Eye Drops For
Travoprost eye drops are prescribed to lower eye pressure, helping to safeguard against vision loss caused by conditions like glaucoma and other eye diseases.
What Is Travoprost Ophthalmic Solution Used For
Travoprost eye drops are prescribed to help manage open-angle glaucoma and high eye pressure by reducing pressure.
What Is Travoprost Used For
Travoprost is commonly prescribed to lower pressure inside the eyes in individuals diagnosed with open-angle glaucoma or ocular hypertension.
Which Condition Is Indicated For A Patient Presenting A Prescription For Travoprost To Be Filled?
The prescription recommends a treatment for open-angle glaucoma or ocular hypertension.