Mounjaro Injection, Tirzepatide
- 1. Introduction to Mounjaro Injection (Tirzepatide)
- 2. Composition and Mechanism of Action
- 3. Approved Medical Uses of Mounjaro Injection
- 4. Off-Label and Investigational Uses of Tirzepatide
- 5. Mounjaro Dosage and Administration Guidelines
- 6. Common and Expected Side Effects of Mounjaro
- 7. Mounjaro Long-term Side effects
- 8. Contraindications for Mounjaro Use
- 9. Important Warnings and Precautions
- 10. Careful Administration and Monitoring
- 11. Use in Special Populations
- 12. Overdose Management
- 13. Drug and Food Interactions
- 14. Storage and Stability Information
- 15. Handling and Safety Precautions
1. Introduction to Mounjaro Injection (Tirzepatide)
Overview of Mounjaro and Its Development
Mounjaro, generically known as tirzepatide, represents a groundbreaking advancement in metabolic medicine. Developed by Eli Lilly, this novel injectable therapy harnesses dual incretin activity to manage blood glucose and body weight. Mounjaro introduces a new class of glucose-lowering agents that go beyond traditional monotherapy, offering robust clinical outcomes for patients with type 2 diabetes.

Approval Status by FDA and Other Health Authorities
The U.S. Food and Drug Administration (FDA) approved Mounjaro in May 2022 for the treatment of type 2 diabetes. Since then, global regulatory bodies, including the European Medicines Agency (EMA), have followed suit, acknowledging its efficacy and safety profile. Additional approvals for obesity treatment are under review.
Marketed Names and Manufacturer
Mounjaro is the brand name under which tirzepatide is sold. Manufactured by Eli Lilly and Company, the medication is available in several countries as a pre-filled pen injection.
Comparison with Other GLP-1 and GIP Receptor Agonists
Unlike conventional GLP-1 receptor agonists like semaglutide and liraglutide, tirzepatide also targets the glucose-dependent insulinotropic polypeptide (GIP) receptor. This dual action amplifies metabolic benefits, making it superior in both glycemic control and weight loss in head-to-head clinical trials.
2. Composition and Mechanism of Action
Active Ingredient: Tirzepatide
Each Mounjaro injection contains tirzepatide, a synthetic peptide analogue that mimics the actions of endogenous incretin hormones.
Pharmacological Class: Dual GIP and GLP-1 Receptor Agonist
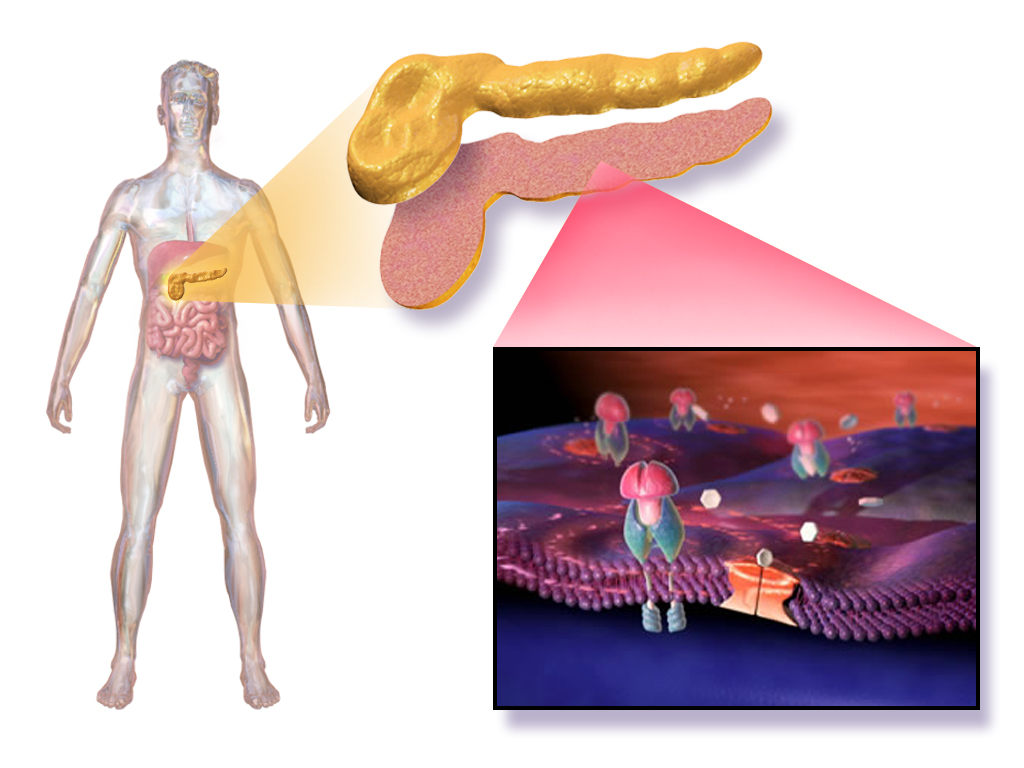
Tirzepatide belongs to a novel class of dual incretin receptor agonists. By stimulating both GIP and GLP-1 pathways, it promotes insulin secretion, suppresses glucagon release, and delays gastric emptying.
Mechanism of Action in Regulating Glucose and Appetite
Tirzepatide acts in the pancreas to enhance insulin secretion in a glucose-dependent manner. In the brain and gastrointestinal tract, it reduces appetite and delays nutrient absorption, contributing to significant weight loss and improved satiety.

Metabolic Pathways and Half-Life
Tirzepatide is primarily degraded by proteolytic enzymes and exhibits a half-life of approximately five days, enabling once-weekly dosing.
Comparison with Semaglutide, Liraglutide, and Dulaglutide
While semaglutide and liraglutide activate GLP-1 receptors alone, tirzepatide's dual receptor action yields greater HbA1c reductions and more profound weight loss. It also shows a more favorable effect on lipid parameters and insulin sensitivity.
Mounjaro vs Ozempic
Mounjaro is known to be more effective in reducing blood sugar levels over a three-month period and aiding in weight loss compared to Ozempic; however, Ozempic has received FDA approval for reducing heart-related issues in individuals with type 2 diabetes and cardiovascular disease.
Zepbound vs mounjaro
Mounjaro is approved for type 2 diabetes in adults with weight loss as a secondary benefit. Zepbound is FDA-approved for weight management in adults who have obesity.
Wegovy vs mounjaro
Wegovy (semaglutide) is approved for managing long-term weight in adults and teens with obesity or being overweight; on the other hand, Mounjaro (tirzepatide) is primarily approved for addressing type 2 diabetes concerns. However, Mounjaro is frequently utilized outside recommendations for weight loss purposes since it showed results in clinical studies.
Trulicity vs mounjaro
Trulicity has been authorized to assist in reducing the likelihood of heart-related issues in adults who have Type 2 diabetes. Mounjaro is more effective in managing blood glucose levels compared to Trulicity. Additionally, Mounjaro may aid in achieving weight loss; however, it's important to note that neither of these medications is sanctioned for weight reduction purposes.
Semaglutide vs mounjaro
Semaglutide and Mounjaro are both types of drugs that assist in managing blood sugar levels and facilitating weight loss; however, their mechanisms differ. Semaglutide functions as a GLP-1 receptor agonist by imitating a hormone, whereas Mounjaro operates as a dual GIP and GLP-1 receptor agonist that targets two distinct hormones with the potential for more significant impacts in regulating blood sugar levels and promoting weight loss
3. Approved Medical Uses of Mounjaro Injection
Type 2 Diabetes Mellitus (Glycemic Control)
Mounjaro is indicated for adults with type 2 diabetes to improve glycemic control as an adjunct to diet and exercise. It is especially effective in individuals with poor response to first-line therapies such as metformin.
Cardiovascular Risk Reduction in Diabetics (Emerging Evidence)
4. Off-Label and Investigational Uses of Tirzepatide
Mounjaro Weight loss
Tirzepatide has demonstrated significant weight reduction in non-diabetic individuals with obesity. Clinical trials like SURMOUNT-1 confirm its potential as a powerful anti-obesity agent.

Mounjaro weight loss by week
Studies indicate that individuals typically experience a 1% reduction in weight per week during the four weeks of their weight loss journey; however, this rate may vary and is influenced by individual factors. A separate research study revealed that individuals on a 15mg maintenance dosage for a period of 72 weeks saw a weight loss of up to 22.4%
Polycystic Ovary Syndrome (PCOS)
Non-Alcoholic Fatty Liver Disease (NAFLD) and NASH
Insulin Resistance Syndromes
Potential Cognitive and Neuroprotective Applications
Animal models suggest that incretin therapies may exhibit neuroprotective effects. Tirzepatide's role in cognitive disorders such as Alzheimer's disease is currently under investigation.
5. Mounjaro Dosage and Administration Guidelines
Recommended Starting Dose and Titration Schedule
Initial dose: 2.5 mg subcutaneously once weekly. Titrate every four weeks to 5 mg, 7.5 mg, 10 mg, 12.5 mg, or a maximum of 15 mg based on tolerability and glycemic response.
Mounjaro Dose for weight loss
The recommended dosage of Mounjaro for weight reduction is 2.5 mg per week over a period of four weeks.
Mounjaro Doses
If gastrointestinal symptoms are pronounced, titration may be slowed. Dose reductions may be considered if adverse effects persist or for frail patients.
Mounjaro Injection sites
Administered in the abdomen, thigh, or upper arm. Rotate injection sites to minimize local irritation.
Mounjaro Dosing Schedule
Administer once weekly, on the same day each week, with or without food. Time of day is flexible.
Best time of day to take mounjaro
Injecting doses at a time, every week, is important, regardless of the time of day you choose to do so.
Tips for Proper Self-Administration
- Use prefilled, single-dose pens
- Ensure the solution is clear and colorless
- Avoid injecting into moles, scars, or irritated skin
Missed Dose Protocol
If a dose is missed, administer within 4 days. If more than 4 days have passed, skip the dose and continue with the next scheduled injection.
6. Common and Expected Side Effects of Mounjaro
Gastrointestinal Effects: Nausea, Vomiting, Diarrhea, Constipation
The most frequent side effects are dose-dependent and typically transient. Symptoms often resolve within weeks of continued use.
Reduced Appetite and Food Aversion
Many users report decreased hunger, which can lead to substantial caloric reduction and weight loss. This may cause aversion to high-fat or heavy meals.
Injection Site Reactions
Localized redness, itching, or swelling may occur, usually mild and self-limiting.
Fatigue or Dizziness
Occasional fatigue or lightheadedness may occur, particularly during the early titration phase.

Timing and Duration of Side Effect Onset
Most side effects present within the first 2-4 weeks and tend to subside with continued treatment.
7. Mounjaro Long-term Side effects
Pancreatitis and Gallbladder Disease
Cases of acute pancreatitis have been reported. Gallbladder issues such as cholelithiasis may also arise due to rapid weight loss.
Hypoglycemia (Especially with Sulfonylureas or Insulin)
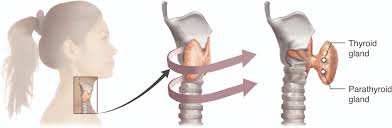
Thyroid C-Cell Tumors: Preclinical Evidence
Rodent studies show increased incidence of C-cell thyroid tumors. Although relevance to humans is uncertain, patients should be monitored for symptoms of thyroid enlargement.
Renal Impairment Secondary to Dehydration
Significant fluid loss from vomiting or diarrhea may precipitate acute kidney injury in vulnerable populations.
Mounjaro side effects hair loss
Although Mounjaro (also known as trizepatide) does not directly result in hair loss on its own, a swift decrease in weight due to the medication may occasionally induce an issue known as telogen effluvium, which can cause an increase in hair shedding.

Hypersensitivity and Anaphylaxis
Severe allergic reactions including angioedema and anaphylaxis have been rarely reported.
Mental Health Disturbances (Mood, Suicidality)
Though uncommon, mood alterations, anxiety, and suicidal ideation warrant immediate medical attention.
8. Contraindications for Mounjaro Use
Known Hypersensitivity to Tirzepatide or Any Excipients
Patients with documented hypersensitivity reactions should not receive Mounjaro.
Personal or Family History of Medullary Thyroid Carcinoma (MTC)
Contraindicated due to theoretical risk based on animal studies.
Multiple Endocrine Neoplasia Syndrome Type 2 (MEN 2)
Tirzepatide should not be used in individuals with this genetic cancer syndrome.
Severe Gastrointestinal Disease (e.g., Gastroparesis)
Mounjaro may worsen symptoms or delay gastric emptying further in patients with preexisting motility disorders.
9. Important Warnings and Precautions
Risk of Thyroid Tumors: Boxed Warning Details
Tirzepatide carries a boxed warning regarding thyroid C-cell tumors. Routine neck palpation and monitoring for symptoms such as dysphagia or hoarseness are advised.
Gastrointestinal Tolerability and Escalation Considerations
To minimize GI symptoms, dose titration should be gradual and closely monitored, particularly in elderly or frail patients.
Fluid and Electrolyte Imbalance
Persistent vomiting or diarrhea can disrupt electrolyte homeostasis, necessitating monitoring in at-risk individuals.
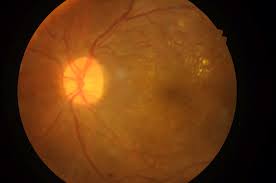
Visual Disturbances in Diabetics
Rapid improvements in glycemic control can temporarily worsen diabetic retinopathy. Baseline and periodic eye exams are recommended.
Delay in Gastric Emptying and Effect on Oral Drug Absorption
Slowed gastric emptying may alter the absorption of oral medications. Caution is advised when co-administering critical therapies like antibiotics, anticonvulsants, or oral contraceptives.
Mounjaro alternatives
10. Careful Administration and Monitoring
Initiation in Patients with Renal or Hepatic Impairment
Tirzepatide should be initiated with caution in individuals with renal or hepatic dysfunction. Although no dosage adjustment is currently recommended for mild to moderate impairment, clinical monitoring is essential due to the risk of dehydration-induced renal decline or hepatic comorbidities common in type 2 diabetes.
Careful Use in Patients with Gastrointestinal Disorders
Given its action in slowing gastric emptying, Mounjaro may exacerbate conditions such as gastroparesis or chronic pancreatitis. Patients with significant gastrointestinal disease should be evaluated before initiating therapy, and alternative agents should be considered where warranted.
Monitoring HbA1c, Renal Function, and Body Weight
Regular assessment of:
- HbA1c: To evaluate long-term glycemic control
- Serum creatinine and eGFR: To detect early renal dysfunction
- Body weight: To track effectiveness in weight reduction and ensure safe weight loss trajectory
is critical to ensure therapeutic success and avoid complications.
Management of Nausea and Dietary Advice
Nausea, a common side effect, can be mitigated through:
- Small, frequent meals low in fat and fiber
- Avoidance of greasy or spicy foods
- Hydration with electrolyte-balanced fluids
Dietary counseling may improve adherence and enhance patient comfort during dose escalation.
11. Use in Special Populations
11.1 Elderly Patients
Pharmacokinetics in Older Adults
Age-related changes in renal clearance and metabolic function do not significantly alter tirzepatide's pharmacokinetics, but increased sensitivity to adverse effects must be anticipated.
Frailty Considerations and Fall Risk
Rapid weight loss and decreased food intake in frail elderly patients can increase fall risk, orthostatic hypotension, and sarcopenia. Individualized care plans are essential.
Dose Adjustment Recommendations
Initiation at the lowest effective dose and slower titration intervals may reduce the risk of intolerance in geriatric patients.
11.2 Pregnant and Breastfeeding Women
Reproductive Toxicity Data from Animal Studies
Animal studies have indicated potential harm to the fetus, including skeletal malformations and growth retardation. As a result, tirzepatide is not recommended during pregnancy unless the potential benefit outweighs the potential risk.
Use Only if Potential Benefit Justifies Potential Fetal Risk
In women with poorly controlled diabetes where alternatives are unsuitable, the decision to use tirzepatide should be made with extreme caution under specialist guidance.
Guidance on Contraception During Treatment
Effective contraception is advised for women of reproductive potential during treatment and for at least four weeks after the last dose.
Excretion into Breast Milk: Data and Recommendations
It is not known whether tirzepatide is excreted in human milk. Due to the potential for adverse reactions in nursing infants, breastfeeding is not recommended during treatment.
11.3 Pediatric Use
Safety and Efficacy in Individuals Under 18 Years
Tirzepatide has not been approved for use in individuals under 18 years of age. Safety and efficacy data in this population remain limited.
Ongoing Clinical Trials in Pediatric Obesity
Several studies are underway to investigate tirzepatide's role in managing adolescent obesity and prediabetes, with results expected to guide future regulatory approval.
12. Overdose Management
Symptoms of Tirzepatide Overdose
Overdose may present with prolonged hypoglycemia, severe gastrointestinal symptoms (nausea, vomiting, diarrhea), and signs of dehydration or electrolyte imbalance.
Supportive Care and Monitoring Strategies
Management includes:
- Intravenous fluids for rehydration
- Frequent blood glucose monitoring
- Administration of dextrose or glucagon if hypoglycemia is present
- Observation for delayed gastrointestinal symptoms or renal compromise
Contacting Poison Control and Emergency Management
In any suspected overdose scenario, prompt contact with local poison control centers and initiation of emergency medical care is imperative.
13. Drug and Food Interactions
Effect on Oral Medications with Narrow Therapeutic Index
Due to delayed gastric emptying, tirzepatide may affect the absorption of oral drugs like:
Close monitoring of therapeutic levels is recommended.
Interaction with Sulfonylureas and Insulin
When combined with sulfonylureas or insulin, there is a heightened risk of hypoglycemia. A reduction in the dose of concurrent antidiabetic agents may be required.
Reduced Appetite and Nutrient Intake Interactions
Significant appetite suppression may inadvertently reduce essential nutrient intake. Nutritional counseling and supplementation may be necessary for long-term users.
Mounjaro and alcohol
Alcohol may increase the risk of hypoglycemia and gastrointestinal irritation. Patients should limit or avoid alcohol while receiving tirzepatide therapy.
14. Storage and Stability Information
Recommended Storage Conditions (Temperature, Light, Moisture)
Store Mounjaro pens in a refrigerator at 2°C to 8°C (36°F to 46°F). Avoid exposure to direct light and high humidity. Do not freeze.
Shelf Life After Opening or Removal from Refrigeration
Once removed from refrigeration, the pen may be kept at room temperature (not above 30°C/86°F) for up to 21 days. Discard if not used within this period.
Safe Disposal of Pens and Needles
Used pens and needles should be disposed of in a puncture-resistant sharps container. Follow local regulations for pharmaceutical waste disposal.
15. Handling and Safety Precautions
Instructions for Healthcare Professionals and Caregivers
- Verify proper injection technique during patient education
- Confirm patient understanding of titration schedule
- Ensure proper storage and transport during travel or extended use
Needle Safety and Sharps Disposal
Never reuse or share needles. Dispose of used sharps safely to prevent needle-stick injuries or transmission of bloodborne pathogens.
Avoiding Contamination and Product Damage
Do not shake or freeze the pen. Inspect visually for discoloration or particulates before use. If the solution is cloudy or contains particles, do not administer.
Mounjaro Injection, Tirzepatide FAQ
- What is the Mounjaro injection for?
- Is Mounjaro effective for weight loss?
- Is Mounjaro approved for weight loss?
- Who cannot take Mounjaro?
- Can I get Mounjaro without diabetes?
- Can Mounjaro reduce belly fat?
- Who is eligible for Mounjaro?
- What are Mounjaro's side effects?
- Can I take Mounjaro to lose 5 kilos?
- Which is better, Ozempic or Mounjaro?
- Is Mounjaro safe for the liver?
- What happens when you stop taking Mounjaro?
- Can Mounjaro cause hair loss?
- Can I buy Mounjaro over the counter?
- How long do you stay on Mounjaro for weight loss?
- How much weight can I lose in 3 months on Mounjaro?
- Where is the best place to inject Mounjaro?
- What are the long term side effects of Mounjaro?
- What happens when you stop Mounjaro?
- Can Mounjaro cause heart problems?
- Are Mounjaro injections safe?
- Can I lose 30 kg on Mounjaro?
- Do you have to take Mounjaro forever?
- Can I lose weight without Mounjaro?
- What is the generic name for Mounjaro?
- What is the best alternative to Mounjaro?
- Who cannot take Mounjaro?
- Why are you cold on Mounjaro?
- Who is not eligible for Mounjaro?
- How long can I stay on Mounjaro?
- Where do you inject Mounjaro?
- Can Mounjaro cause long-term effects?
- Does Mounjaro cause kidney problems?
- Does Mounjaro have a pill form?
- Does Mounjaro cause sleep issues?
- Is Mounjaro safe for weight loss?
- Will I gain weight back if I stop Mounjaro?
- Can I lose 10kg in a month with Mounjaro?
- What happens if you inject Mounjaro in the wrong place?
- What does Mounjaro do to your body?
- What foods should you avoid when taking Mounjaro?
- Can you stay on Mounjaro for life?
- How quickly do you lose weight on Mounjaro?
- Does Mounjaro have heart and kidney benefits?
- Is it better to inject Mounjaro in the stomach or thigh?
- Do you regain weight after stopping Mounjaro?
- How much weight can I lose in 3 months on tirzepatide?
- How to use mounjaro injection?
- How to give mounjaro injection?
- Does the Mounjaro injection hurt?
- What is the best injection site for mounjaro?
- How to do mounjaro injection?
- Why does my mounjaro injection site itch?
- Does injection site matter for mounjaro?
- How long after mounjaro injection do side effects start?
- Where to give mounjaro injection?
- What are the side effects of mounjaro injection?
- Can I change my mounjaro injection day?
- How quickly does Mounjaro work?
- Does Mounjaro make you sleepy?
- What medicines can you not take with Mounjaro?
What is the Mounjaro injection for?
Mounjaro is a medication that can be injected and is intended for adults with type 2 diabetes. It is typically taken in combination with a diet and regular physical activity to help enhance blood sugar levels (glucose). However, it's uncertain whether Mounjaro is safe for individuals who have previously experienced pancreatitis, an inflammation of the pancreas.
Is Mounjaro effective for weight loss?
Mount Kilimanjaro helped individuals struggling with obesity shed more than 20% of their body weight.
Is Mounjaro approved for weight loss?
Mount Kilimanjaro is not endorsed for weight reduction purposes; however, tirzepatide (the ingredient in it) has gained approval for weight loss treatment.
Who cannot take Mounjaro?
Individuals with a background of medullary thyroid carcinoma (also known as MTC), whether within their family or personal history, or those diagnosed with Multiple Endocrine Neoplasia syndrome type 2
Can I get Mounjaro without diabetes?
It is commonly prescribed for individuals with diabetes; however, medical professionals may also suggest its use for treating conditions such as obesity, off-label.
Can Mounjaro reduce belly fat?
Mount Kilimanjaro is known for its effectiveness in reducing accumulation in the body.
Who is eligible for Mounjaro?
Having a body mass index (BMI ) of 40 kg/m² or higher (or 37.2 kg/m², for individuals from minority backgrounds)
What are Mounjaro's side effects?
Common Mounjaro side effects include digestive distress, such as vomiting, diarrhea, constipation and nausea.
Can I take Mounjaro to lose 5 kilos?
Yes
Which is better, Ozempic or Mounjaro?
In general, Mounjaro is considered more effective than Ozempic in aiding weight loss and lowering blood sugar levels (as measured by a reduction in hemoglobin A1c) in individuals with type 2 diabetes.
Is Mounjaro safe for the liver?
Yes
What happens when you stop taking Mounjaro?
As the medication's effects linger for 5 days in your system, you can expect an increase in hunger rather than a sudden surge. For some individuals, readjusting to their appetite levels can pose a challenge.
Can Mounjaro cause hair loss?
Many individuals mention experiencing hair thinning once they begin using Mounjaro; this is probably a result of weight reduction rather than the medication's direct effects.
Can I buy Mounjaro over the counter?
No
How long do you stay on Mounjaro for weight loss?
72 weeks
How much weight can I lose in 3 months on Mounjaro?
9% of body fat
Where is the best place to inject Mounjaro?
- The stomach area (abdomen)
- The from part of the thigh
- The upper arm
What are the long term side effects of Mounjaro?
Pancreatitis, gallbladder problems, kidney problems or allergic reactions
What happens when you stop Mounjaro?
Your appetite can bounce back to its normal state rapidly.
Can Mounjaro cause heart problems?
Yes
Are Mounjaro injections safe?
When Mounjaro is taken as directed and used properly for weight loss purposes, it proves to be a beneficial treatment.
Can I lose 30 kg on Mounjaro?
Yes
Do you have to take Mounjaro forever?
No
Can I lose weight without Mounjaro?
Yes
What is the generic name for Mounjaro?
Tirzepatide
What is the best alternative to Mounjaro?
Ozempic (semaglutide), Rybelsus (semaglutide), and Trulicity (dulaglutide)
Who cannot take Mounjaro?
- History of medullary thyroid carcinoma (MTC)
- Patients with Multiple Endocrine Neoplasia syndrome type 2
Why are you cold on Mounjaro?
Mountains can help reduce blood pressure levels and promote heart health; however, it may result in reduced blood flow to the extremities, like hands and feet, which can lead to a sensation of coldness due to reduced blood circulation while the body focuses on maintaining core organ function.
Who is not eligible for Mounjaro?
If your BMI is below 40
How long can I stay on Mounjaro?
2 years
Where do you inject Mounjaro?
The medication Mounjaro is administered beneath the skin in areas, like your stomach, thigh, or upper arm.
Can Mounjaro cause long-term effects?
Yes
Does Mounjaro cause kidney problems?
Other GLp receptor agonists have been associated with kidney issues.
Does Mounjaro have a pill form?
No
Does Mounjaro cause sleep issues?
No
Is Mounjaro safe for weight loss?
Tirzapetide is the component in Mounjaro that has been approved by the FDA not just for addressing type 2 diabetes but also for aiding with weight loss.
Will I gain weight back if I stop Mounjaro?
Yes
Can I lose 10kg in a month with Mounjaro?
In clinical studies, it was found that typically one could shed 4 percent of their weight in a month and up to 6 percent in two months.
What happens if you inject Mounjaro in the wrong place?
There is no proof that the efficiency of Mounjaro varies with the location of injection sites.
What does Mounjaro do to your body?
This medicine functions by stimulating two receptors known as GLP-1 and GIP to enhance the presence of incretins – hormones. It operates by supporting your body in generating insulin when necessary. It also diminishes the quantity of glucose or sugar produced by the liver and decelerates the pace at which food is digested.
What foods should you avoid when taking Mounjaro?
Foods high in sugar and unhealthy fats
Can you stay on Mounjaro for life?
No
How quickly do you lose weight on Mounjaro?
4 weeks
Does Mounjaro have heart and kidney benefits?
The advantages of Mounjaros' heart health properties involve decreasing blood pressure and improving cholesterol levels, as well as alleviating symptoms of heart failure in individuals with preserved ejection fraction (HF pEF).
Is it better to inject Mounjaro in the stomach or thigh?
The amount of tirzapetide in your blood stays the same no matter where you administer it.
Do you regain weight after stopping Mounjaro?
Yes
How much weight can I lose in 3 months on tirzepatide?
10 pounds
How to use mounjaro injection?
Remember to select where you'll inject carefully with the guidance of your healthcare provider. Remove the cap from the base and position it flat against your skin; then unlock it by turning. Hold down the button for a maximum of 10 seconds while listening attentively for the clicking sound.
How to give mounjaro injection?
Remember to select where you'll inject carefully with the guidance of your healthcare provider. Remove the cap from the base and position it flat against your skin; then unlock it by turning. Hold down the button for a maximum of 10 seconds while listening attentively for the clicking sound.
Does the Mounjaro injection hurt?
No
What is the best injection site for mounjaro?
abdomen
How to do mounjaro injection?
Remember to select the injection site carefully, following the guidance of your healthcare provider. Remove the cap from the base and position it flat against your skin; then unlock it by turning. Hold down the button for a maximum of 10 seconds while listening attentively for the clicking sound.
Why does my mounjaro injection site itch?
The skin's response to needles is an natural reaction.
Does injection site matter for mounjaro?
No
How long after mounjaro injection do side effects start?
If you encounter any side effects after receiving a vaccination or adjusting the dosage level of the medication for the time; these effects are likely to manifest thereafter.
Where to give mounjaro injection?
skin (subcutaneously) of your stomach (abdomen), thigh, or upper arm
What are the side effects of mounjaro injection?
- Nausea
- Diarrhoea
- Indigestion
- Vomiting
- Constipation
- Stomach pain
- Alopecia
Can I change my mounjaro injection day?
Yes
How quickly does Mounjaro work?
Within hours of your first injection
Does Mounjaro make you sleepy?
Fatigue is an issue when using Mounjaro as it can lead to fluctuating energy levels due to reduced calorie intake. This results in your body having less fuel than usual, which may cause it to slow down slightly in order to conserve energy.
What medicines can you not take with Mounjaro?
Other Diabetes medications, Blood sugar medications (e.g Metformin), Warfarin, Digoxin, Birth control medication, Thyroid medications, Painkillers