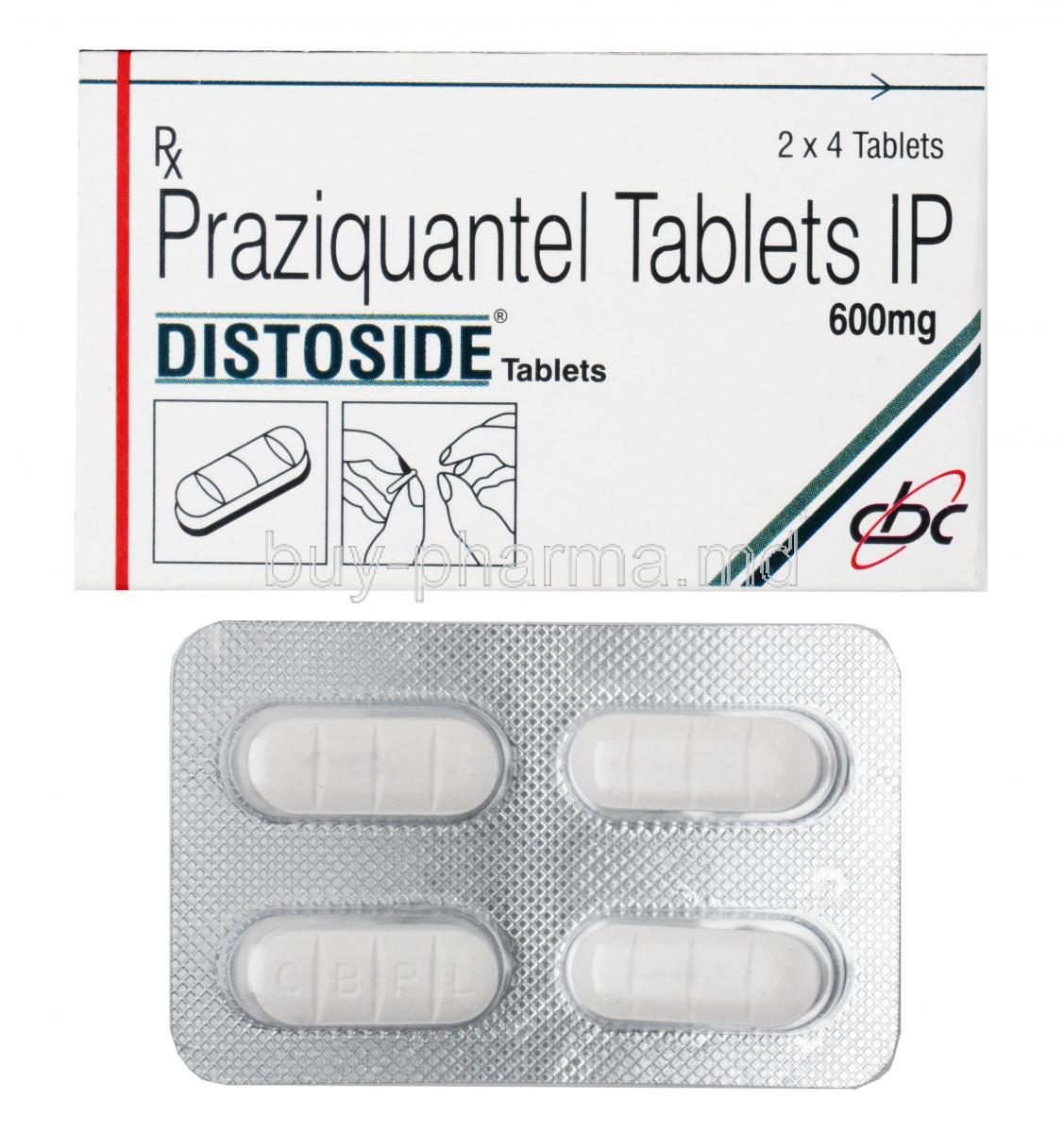
Praziquantel
Uses
Distoside is used in the treatment of human parasite infections, schistosoma infections.
How it Works
Distoside is known as an anthelmintic. Distoside works for human parasite infections by stopping the insect larvae from growing and spreading. Distoside affects the permeability of the cell membrane by making it weaker thereby killing the worms.
Common Side effects
Patients who take Distoside may suffer from such side effects;
Headache,
Nausea,
Vomiting,
Abnormal liver function tests,
Hair loss,
Fever,
Dizziness,
Abdominal pain,
Vertigo