Wegovy Pen
- 1. Introduction to Wegovy Pen
- 2. Wegovy Ingredients
- 3. Medical Uses of Wegovy Pen
- 4. Off-Label and Investigational Uses
- 5. How Wegovy Works in the Body
- 6. Dosage and Administration Guidelines
- 7. Common and Serious Side Effects of Wegovy
- 8. Drug Interactions and Contraindications
- 9. Warnings and Important Precautions
- 10. Special Considerations for Use
- 11. Careful Administration and Monitoring
- 12. Handling and Storage Instructions
- 13. Overdose Management and Emergency Protocol
- 14. Handling Precautions and Patient Counseling Points
1. Introduction to Wegovy Pen
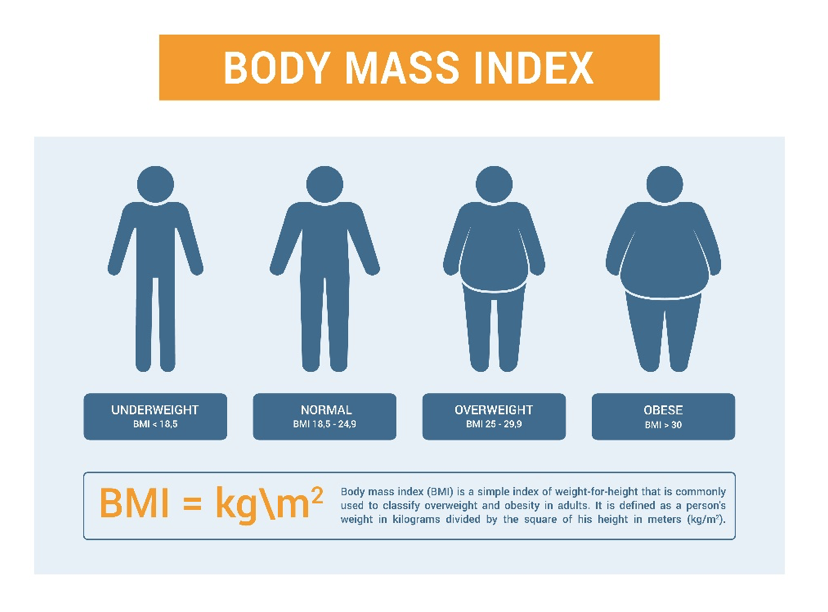
Wegovy Pen is a prescription medication containing semaglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, approved by the U.S. Food and Drug Administration (FDA) for chronic weight management. It is indicated for adults with a body mass index (BMI) of >30 kg/m² (obesity) or >27 kg/m² (overweight) who also have weight-related medical conditions such as hypertension, dyslipidemia, or type 2 diabetes.
Unlike other GLP-1 receptor agonists like Ozempic and Saxenda, Wegovy is specifically developed and dosed for weight loss rather than glycemic control. Its formulation and delivery system are tailored to provide sustained therapeutic effects with once-weekly injections.
Wegovy is approved in multiple regulatory jurisdictions, including the United States, European Union, Canada, and several Asia-Pacific markets. It is available in prefilled single-use pens with dose increments ranging from 0.25 mg to 2.4 mg.
2. Wegovy Ingredients
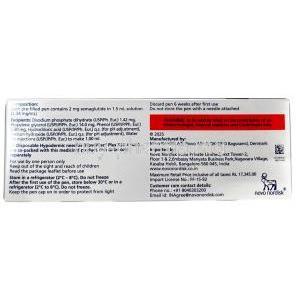
The active pharmaceutical ingredient in Wegovy is semaglutide, a synthetic analog of human GLP-1 with 94% sequence homology. Semaglutide is modified to resist enzymatic degradation, allowing for extended half-life and once-weekly dosing.
- Active ingredient: Semaglutide
- Excipients: Disodium phosphate dihydrate, sodium chloride, and water for injection
Wegovy is administered as a subcutaneous injection. Its extended-release effect is achieved through molecular modifications and formulation science, ensuring stable plasma concentrations over seven days.
Wegovy vs ozempic
Wegovy and Ozempic both contain the same active ingredient, semaglutide, a GLP-1 receptor agonist. The primary difference lies in their approved uses and dosage: Ozempic is primarily for treating Type 2 diabetes and reducing cardiovascular risk in diabetic patients, while Wegovy is specifically approved for chronic weight management at a higher dosage. Both work by mimicking a natural hormone that helps regulate blood sugar, slow digestion, and reduce appetite.
Wegovy vs zepbound
Wegovy (semaglutide) is a GLP-1 receptor agonist, mimicking a single gut hormone to reduce appetite and slow gastric emptying for weight loss. Zepbound (tirzepatide) is a "dual-agonist," targeting both GLP-1 and GIP receptors, which can lead to greater weight loss in clinical trials compared to Wegovy. Both are once-weekly injectable medications approved for chronic weight management in conjunction with diet and exercise.
Saxenda vs wegovy
Saxenda (liraglutide) and Wegovy (semaglutide) are both injectable medications from the GLP-1 receptor agonist class, used for chronic weight management alongside diet and exercise. The key differences are their dosing frequency and efficacy: Saxenda is typically injected daily, while Wegovy is injected once weekly and generally leads to greater average weight loss.
Wegovy vs mounjaro
Wegovy (semaglutide) and Mounjaro (tirzepatide) are both once-weekly injectable medications used for weight management, but they differ in their active ingredients and mechanisms. Wegovy is a GLP-1 receptor agonist, while Mounjaro is a dual GLP-1 and GIP receptor agonist, potentially leading to greater weight loss effects. Wegovy is FDA-approved specifically for chronic weight management, whereas Mounjaro is currently approved for Type 2 diabetes and often used off-label for weight loss.
Tirzepatide vs wegovy
Wegovy (semaglutide) is a GLP-1 receptor agonist, mimicking a gut hormone that helps regulate appetite and satiety. Tirzepatide (found in Mounjaro and Zepbound) is a dual agonist, activating both GLP-1 and GIP receptors, which are two different gut hormones involved in blood sugar control and appetite regulation. This dual action is thought to contribute to tirzepatide generally showing greater weight loss in clinical trials compared to semaglutide.
3. Medical Uses of Wegovy Pen
Wegovy is indicated for long-term weight management in adults with obesity or overweight with at least one weight-related condition. It is prescribed in conjunction with lifestyle interventions including a reduced-calorie diet and increased physical activity.
The duration of therapy depends on individual response and tolerance. Clinical trials have demonstrated sustained weight loss over 68 weeks, with many patients achieving 10-15% body weight reduction.

4. Off-Label and Investigational Uses
In clinical practice and research, Wegovy is being explored for several off-label applications:
- Polycystic Ovary Syndrome (PCOS): Semaglutide shows promise in improving weight, insulin sensitivity, and hormonal balance.
- Appetite suppression in non-diabetics: Used off-label for patients with severe obesity resistant to conventional therapies.
- Addiction and behavioral impulse control: Emerging studies are evaluating its influence on reward pathways in the brain.
- Cardiovascular risk reduction: Its weight-lowering effects may indirectly reduce major adverse cardiovascular events (MACE).
5. How Wegovy Works in the Body
Wegovy mimics endogenous GLP-1, a hormone involved in glucose regulation and appetite control. By activating GLP-1 receptors:
- It slows gastric emptying, prolonging satiety after meals
- Reduces appetite through hypothalamic signaling
- Enhances insulin secretion in a glucose-dependent manner
This multifaceted mechanism leads to decreased caloric intake and sustained weight reduction without significant loss of lean body mass.
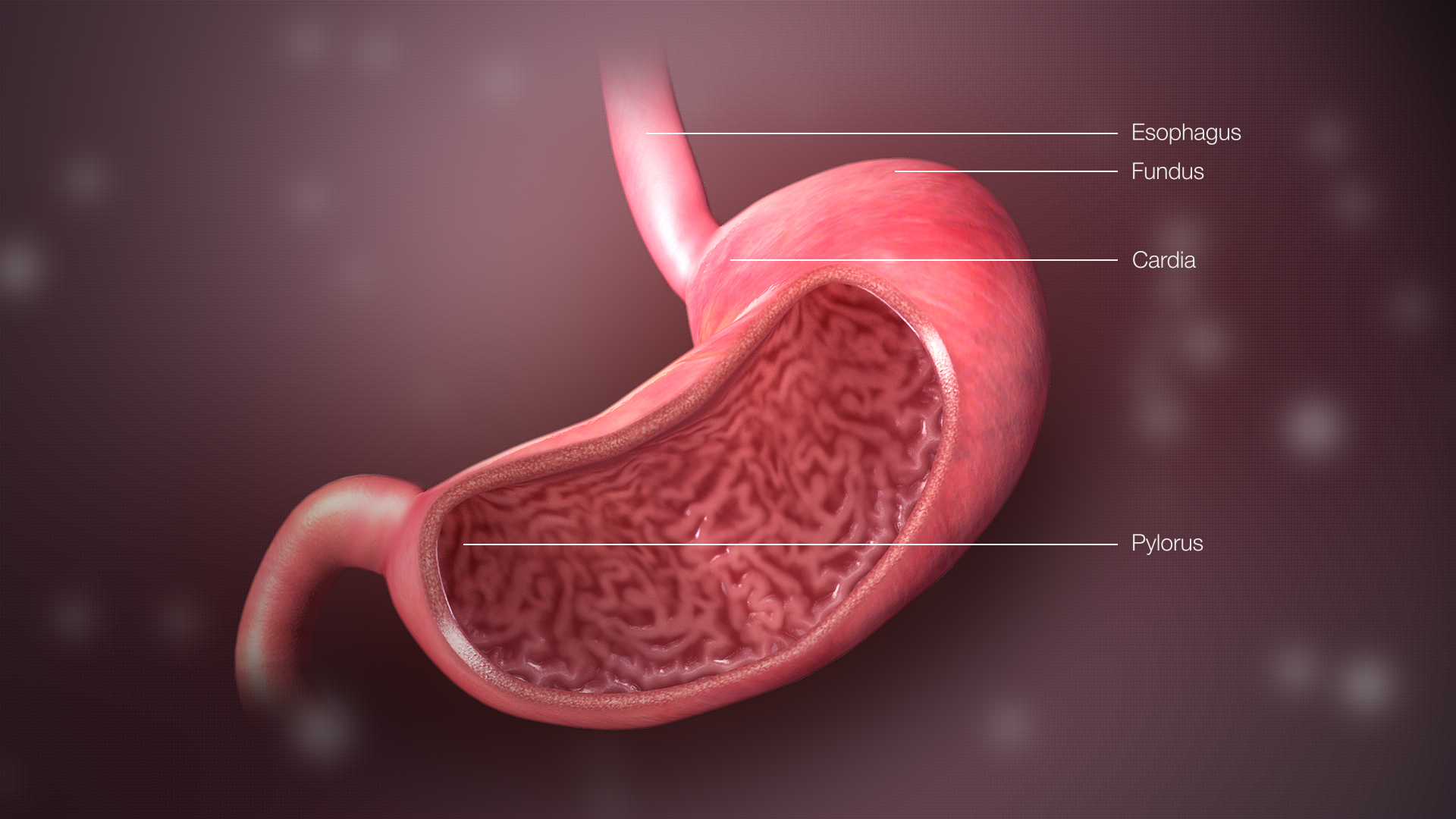
How does Wegovy work for weight loss
Wegovy (semaglutide) aids in weight reduction by imitating a hormone known as GLP 1 that controls appetite and slows the pace at which food passes from the stomach to the intestines. This results in a longer sensation of fullness, leading to decreased food consumption and, ultimately, shedding pounds.

Wegovy weight loss results
In trials, at hospitals and healthcare facilities, the majority who used Wegovy at doses of 1.7 mg and 2.4 mg experienced weight loss ranging from 5 percent to 15 percent over a period spanning 68 weeks on average. In a study conducted in the year 2022 involving individuals grappling with weight or obesity, there was an average weight reduction of 5.9 percent, after three months following the use of semaglutide. By the end of six months post use, this figure had risen to a reduction rate reaching 10.9 percent.
6. Dosage and Administration Guidelines
Wegovy is administered via subcutaneous injection once weekly, ideally on the same day each week. Titration is essential to reduce gastrointestinal side effects:
Wegovy doses and Wegovy dosing schedule
- Week 1-4: 0.25 mg weekly
- Week 5-8: 0.5 mg weekly
- Week 9-12: 1.0 mg weekly
- Week 13-16: 1.7 mg weekly
- Maintenance dose: 2.4 mg weekly from week 17 onwards
If a dose is missed, it should be taken within 5 days. If more than 5 days have passed, the next dose should be skipped, and regular weekly administration resumed.
How to take WeGovy
Instruct them to push the pen down throughout the injection and not to retract it until the yellow bar has come to a stop, moving completely and finished moving on its own. If they encounter difficulties during the injection process, suggest switching to an injection spot, like the upper leg or upper arm, or try injecting while standing up into the lower abdomen or stomach region.
Wegovy injection site
- The belly area (stomach) is a chosen and usually convenient spot for injections; it's advisable to inject a minimum of 2 inches from your navel.
- When targeting the thigh for injections aim for the front of your thigh.
- Although the upper arm can serve as an injection site, individuals might encounter challenges reaching it independently. It may require some help.

7. Common and Serious Side Effects of Wegovy
7.1 Common Adverse Reactions
The most frequently reported side effects are gastrointestinal in nature:
- Nausea and vomiting
- Wegovy Diarrhea or constipation
- Abdominal discomfort, bloating
- Wegovy Headache and fatigue
- Injection site redness or irritation
- Wegovy hair loss

7.2 Wegovy Long-term Side Effects
While rare, serious adverse events may occur:
- Acute pancreatitis: Abdominal pain radiating to the back, with or without vomiting
- Gallbladder disease: Including gallstones or inflammation (cholecystitis)
- Wegovy side effects cancer: Semaglutide has caused C-cell tumors in rodents; relevance in humans remains under investigation
- Kidney injury: Resulting from severe dehydration due to vomiting or diarrhea
8. Drug Interactions and Contraindications
8.1 Drug Interactions
- Wegovy may delay gastric emptying, affecting absorption of oral medications such as antibiotics and contraceptives
- Caution with insulin and sulfonylureas due to increased hypoglycemia risk
- Avoid concomitant use with other GLP-1 agonists to prevent overdose
8.2 Contraindications
- Personal or familial history of medullary thyroid carcinoma (MTC)
- Multiple endocrine neoplasia syndrome type 2 (MEN 2)
- Known hypersensitivity to semaglutide or any formulation component
9. Warnings and Important Precautions
- Thyroid tumor risk: Includes a black box warning due to rodent data suggesting increased incidence of C-cell tumors
- Gastrointestinal disorders: Use cautiously in patients with severe gastroparesis or inflammatory bowel disease
- Hypoglycemia: Especially in combination with insulin or insulin secretagogues
- Retinopathy: Rapid glucose improvement may temporarily worsen diabetic eye disease
Routine monitoring and individualized medical supervision are recommended throughout the course of treatment with Wegovy.
Wegovy side effects with alcohol
Using Wegovy and alcohol together is not recommended as it could amplify side effects, like nausea and dizziness associated with Wegovy treatment. Its advised to avoid alcohol while taking Wegovy to prevent any effects that can hinder your weight loss progress.
Wegovy alternative
10. Special Considerations for Use
10.1 Administration in Elderly Patients
Pharmacokinetic studies indicate that semaglutide exposure in individuals aged 65 years and older is comparable to that in younger adults. Nonetheless, due to age-associated physiological changes, clinical discretion is advised.
- Renal function: Evaluate glomerular filtration rate (GFR) prior to initiation and periodically during treatment, as older patients may have diminished renal reserve.
- Hepatic function: Hepatic impairment may alter semaglutide metabolism; baseline liver function tests are advisable in this population.
- Monitoring: Although no dosage adjustments are routinely required, close observation for gastrointestinal tolerance and weight-related adverse effects is recommended.
10.2 Use in Pregnant and Nursing Women
Wegovy (semaglutide) is classified as a pregnancy category not yet assigned under the FDA's current labeling system, but animal studies have shown embryofetal toxicity at clinically relevant doses.
- Pregnancy: Discontinue Wegovy as soon as pregnancy is detected. Weight loss medications are contraindicated during pregnancy, and semaglutide's effects on fetal development remain unquantified in humans.
- Lactation: It is unknown whether semaglutide is excreted in human breast milk. Due to the potential for serious adverse reactions in nursing infants, use during breastfeeding is not recommended.

10.3 Use in Pediatric Populations
Wegovy is FDA-approved for chronic weight management in pediatric patients aged 12 to 17 years with obesity (BMI >95th percentile for age and sex). This approval follows clinical trial evidence demonstrating meaningful weight reduction and metabolic improvements in adolescents.
- Clinical data: The STEP TEENS trial indicated significant BMI reductions and improved cardiometabolic profiles in this age group.
- Children under 12: Safety and efficacy have not been established in this population. Use in pre-adolescents is not recommended pending further studies.
11. Careful Administration and Monitoring
Wegovy therapy necessitates individualized planning and clinical vigilance. Patient tolerance, clinical response, and metabolic safety must be assessed routinely.
- Target monitoring: Regular evaluation of weight loss progression helps determine therapeutic success. A failure to lose at least 5% of baseline body weight within 16 weeks may prompt discontinuation.
- Adverse effects: Intolerable nausea, vomiting, or dehydration warrant dosage reassessment or cessation.
- Renal and electrolyte status: Monitor serum creatinine and electrolytes, particularly in patients with prior gastrointestinal loss or existing renal impairment.
- Liver enzymes: ALT, AST, and bilirubin should be assessed periodically, especially in patients with hepatic comorbidities or unexplained symptoms.
12. Handling and Storage Instructions
Proper storage and disposal of Wegovy pens are critical for maintaining drug efficacy and ensuring user safety.
- Refrigeration: Store pens in a refrigerator at 2°C to 8°C (36°F to 46°F) before initial use. Do not freeze. Protect from light.
- In-use storage: After first use, pens can be kept at room temperature up to 30°C (86°F) for up to 28 days.
- Disposal: Used pens should be discarded in FDA-cleared sharps containers. Never reuse or share pens to avoid cross-contamination or infection.
13. Overdose Management and Emergency Protocol
Although overdose with semaglutide is rare, excessive administration may provoke pronounced gastrointestinal and metabolic disturbances.
- Symptoms: Nausea, vomiting, severe abdominal pain, and potential hypoglycemia (especially when co-administered with insulin or sulfonylureas).
- Treatment: Supportive care is the cornerstone of overdose management. Ensure adequate hydration and electrolyte balance.
- Antidote: No specific antidote exists for semaglutide overdose. Hospital admission may be required in severe cases.
14. Handling Precautions and Patient Counseling Points
Proper patient education and preventive practices improve treatment outcomes and reduce risks associated with Wegovy therapy.
- Self-injection technique: Patients must be trained on subcutaneous administration in the abdomen, thigh, or upper arm. Rotation of injection sites is recommended.
- Pen hygiene: Never share pens to prevent viral or bacterial transmission. Use each pen for a single patient only.
- Lifestyle adherence: Reinforce the importance of adhering to a balanced diet and regular physical activity for maximum weight loss benefit.
- Symptom vigilance: Advise immediate medical attention if signs of pancreatitis (severe abdominal pain), allergic reactions, or dehydration occur.

Wegovy Pen FAQ
- How many doses of Wegovy are in one pen?
- What is a Wegovy pen for?
- Are Wegovy and Ozempic exactly the same?
- Where do I inject the Wegovy pen?
- How many Wegovy pens do you need per month?
- Are Wegovy pens safe?
- What is the side effect of Wegovy?
- Is Wegovy cheaper than Ozempic?
- Is Wegovy safe?
- What happens if I drop my Wegovy pen?
- How fast does Wegovy work?
- What happens when you stop taking Wegovy?
- How long can you stay on Wegovy?
- Who should not take Wegovy?
- Can I travel with a Wegovy pen?
- How do I know if Wegovy pen worked?
- Is there a generic for Wegovy?
- Does Wegovy cause hair loss?
- How much weight can you lose in a month with Wegovy?
- Does Wegovy cause kidney problems?
- Who qualifies for Wegovy?
- Does Wegovy change your face?
- Does Wegovy affect your organs?
- Do Wegovy pens need to be refrigerated?
- How much weight can you lose in 3 months with Wegovy?
- Is Wegovy 1 pen per week?
- Who shouldn't take Wegovy?
- Is Wegovy safe for non-diabetic patients?
- Can I miss Wegovy for 2 days?
- Where to inject Wegovy?
- How to use the Wegovy pen for the first time?
- Is there a pill version of Wegovy?
- Is Wegovy FDA approved for weight loss?
- What is the most common side effect of Wegovy?
- Does Wegovy actually work?
- How quickly does Wegovy work?
- Can I lose 40 pounds in 2 months on Wegovy?
- Who is a good candidate for Wegovy?
- What organs are affected by Wegovy?
- Is WeGovy covered by insurance?
- Is Wegovy the same as ozempic?
- Where to inject wegovy?
- How to inject Wegeovy?
- Does wegovy make you tired?
- How long do you take wegovy for weight loss?
- Can I start WeGovy at 1.7 mg?
- At what dose does WeGovy start working?
- Why am I not losing weight on WeGoWY?
- Is Zepbound better than Weegy?
- How to administer WeGovy?
- Can you skip a week of WeGovy?
- How long does it take for wegovy to work?
- How long does nausea last with wegovy?
- How quickly does WeGovy work?
How many doses of Wegovy are in one pen?
1 dose
What is a Wegovy pen for?
To assist kids who are 12 and above dealing with obesity in shedding body weight and maintaining a healthy weight.
Are Wegovy and Ozempic exactly the same?
Wegovy and Ozempic are two brand names for the medication semaglutide, with varying purposes; Wegovy is specifically designed to help both adults and children aged 12 and above with obesity manage their weight effectively; meanwhile, Ozempic is intended for regulating blood sugar levels in individuals diagnosed with type 2 diabetes.
Where do I inject the Wegovy pen?
The medication should be administered under the skin, in the stomach area, or in the upper arm.
How many Wegovy pens do you need per month?
4
Are Wegovy pens safe?
Yes
What is the side effect of Wegovy?
- Nausea
- diarrhea
- vomiting
- constipation
- stomach (abdomen) pain
- headache
- tiredness (fatigue)
- upset stomach
- dizziness
- feeling bloated
- belching
- low blood sugar in people with type 2 diabetes
- gas
- stomach flu
- heartburn
Is Wegovy cheaper than Ozempic?
No
Is Wegovy safe?
Wegovy is often seen as an successful method for achieving lasting weight reduction.
What happens if I drop my Wegovy pen?
Make sure not to use your Wegovy pen if it has been dropped since it contains glass components.
How fast does Wegovy work?
You may begin to notice weight loss within the week of using Wegovy.
What happens when you stop taking Wegovy?
Wegovy users typically regained around 2/3 of the weight they had lost once they discontinued the medication regimen.
How long can you stay on Wegovy?
For life
Who should not take Wegovy?
Allergies to ingredients, thyroid cancer or other endocrine conditions, pregnancy, diabetic retinopathy, feelings of depression or suicidal thoughts, pancreatitis, and kidney problems are some health concerns to be mindful of.
Can I travel with a Wegovy pen?
Yes
How do I know if Wegovy pen worked?
When the progress bar comes to a halt the injection process is finished.
Is there a generic for Wegovy?
No
Does Wegovy cause hair loss?
It is not a common side effect.
How much weight can you lose in a month with Wegovy?
Up to 5%
Does Wegovy cause kidney problems?
Wegovys usual side effects, like nausea and vomiting, can cause dehydration issues that may impact the kidneys. The kidneys may not work efficiently when there is dehydration due to blood flow to them.
Who qualifies for Wegovy?
BMI of 30 kg/m² or greater (obesity), BMI of 27 kg/m²-29.9 kg/m² and at least one weight-related comorbid condition., BMI of 27 kg/m² or greater and established CVD.
Does Wegovy change your face?
Yes
Does Wegovy affect your organs?
The liver produces bile that is stored in the gallbladder before being released into the intestine to aid in digesting fats from your food intake. Wegovy has the potential to lead to gallbladder issues necessitating intervention, such as the formation of gallstones (cholelithiasis) or gallbladder inflammation (cholecystitis).
Do Wegovy pens need to be refrigerated?
Yes
How much weight can you lose in 3 months with Wegovy?
Up 6%
Is Wegovy 1 pen per week?
Yes
Who shouldn't take Wegovy?
A family background involving neoplasia type 02 (MEN02) an inherited genetic disorder or a personal or family history of medullary thyroid cancer.
Is Wegovy safe for non-diabetic patients?
Yes
Can I miss Wegovy for 2 days?
Yes
Where to inject Wegovy?
Wegovy is administered weekly by injecting it under the skin, in the stomach area, or upper thigh or upper arm.
How to use the Wegovy pen for the first time?
Rotate the dose selector knob until the control line aligns with the dose counter, ensuring the needle is facing upward then. Hold the administration button until the dose counter resets to zero.
Is there a pill version of Wegovy?
Yes
Is Wegovy FDA approved for weight loss?
Yes
What is the most common side effect of Wegovy?
Nausea
Does Wegovy actually work?
People lost on average 15% of their body weight.
How quickly does Wegovy work?
Wegovy begins to take effect right after the initial dose is administered, but noticeable weight loss outcomes may not be immediate.
Can I lose 40 pounds in 2 months on Wegovy?
Most individuals typically shed 2% of their body mass within a month of using Wegovy.
Who is a good candidate for Wegovy?
It works well for individuals with a body mass index (BMI) of 30 or above or a BMI of 27 or higher combined with health issues like diabetes, high blood pressure, or elevated cholesterol levels.
What organs are affected by Wegovy?
Pancreas, Kidney, Gall bladder, stomach
Is WeGovy covered by insurance?
The insurance coverage provided by Wegovy can vary greatly. It is not always guaranteed.
Is Wegovy the same as ozempic?
Both Ozempic and Wegovy are brand names for a medication called semaglutide; however, their purposes vary slightly. Wegovy is sanctioned for weight management in adults and adolescents aged 12 and above with obesity while Ozempic is authorized for reducing blood sugar levels in individuals with type 2 diabetes.
Where to inject wegovy?
Patients will administer their Wegovy dosage by injecting it under the skin of their arms or stomach (ensuring a 2 inch distance from the belly button) or in the legs (on the front of the thighs).
How to inject Wegeovy?
Position the pen in a line against your body to ensure visibility through the pen's window before pressing against your skin until you hear a clicking sound indicating the start of the injection process. Observe the movement of the bar within the pens window; if it remains stationary apply pressure to ensure proper administration of medication.
Does wegovy make you tired?
Yes
How long do you take wegovy for weight loss?
3 months
Can I start WeGovy at 1.7 mg?
No
At what dose does WeGovy start working?
.25mg
Why am I not losing weight on WeGoWY?
You require time · You should follow a diet with fewer calories · Engage in more physical activity · Stress is impeding your advancement.
Is Zepbound better than Weegy?
The Zepbound resulted in a decrease in waist circumference.
How to administer WeGovy?
Ask them to press the pen throughout the injection and advise against removing the pen until the yellow bar ceases to move.
Can you skip a week of WeGovy?
It's not advisable to miss a week's dose of Wegovy since it could interfere with how the medication works for you. Consistently taking Wegovy each week helps keep levels stable in your system. Skipping a dose might hamper appetite management and slow down your weight loss journey
How long does it take for wegovy to work?
Several weeks
How long does nausea last with wegovy?
A few days
How quickly does WeGovy work?
Upon beginning Wegovy intake, days may trigger a change in your system; however, it might take a couple of weeks before you truly perceive its complete impact on you. Research indicates that certain individuals may potentially shed 2 percent of their body weight during the initial four weeks of starting treatment with the minimal starting dosage.