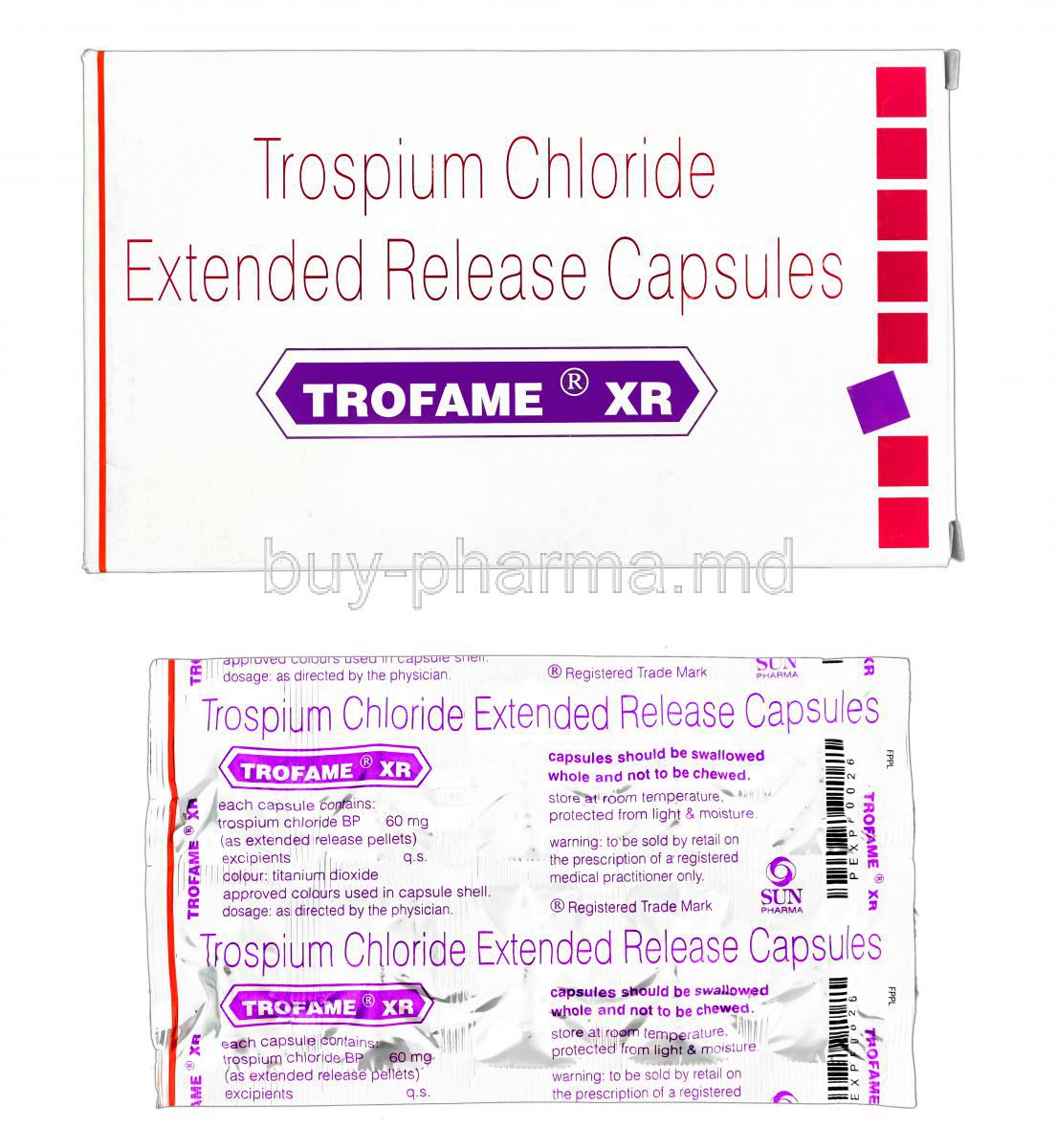
Introduction to Trofame XR
Overview of Trospium Chloride as an Antimuscarinic Agent
Trofame XR contains trospium chloride, a potent antimuscarinic compound designed to modulate overactive bladder activity. By antagonizing muscarinic receptors in the bladder wall, it diminishes involuntary contractions that often lead to urinary urgency and incontinence.
Extended-Release Formulation and Therapeutic Relevance
The extended-release formulation ensures a steady therapeutic effect across the day, minimizing fluctuations in plasma levels. This design promotes improved compliance and reduces the frequency of dosing compared to immediate-release alternatives.
Trospium brand name
Trofame XR is clinically approved for the management of overactive bladder (OAB), characterized by urinary frequency, urgency, and urge incontinence. Its use has been validated by multiple regulatory authorities for long-term control of bladder instability.
Composition and Formulation
Active Ingredient: Trospium Chloride
The core active pharmaceutical substance is trospium chloride, an established antimuscarinic agent with proven efficacy in urological disorders.
Trospium drug class
Trospium is in a class of medications called antimuscarinics. It works by relaxing the bladder muscles to prevent urgent, frequent, or uncontrolled urination.
Extended-Release Mechanism of Trofame XR
The extended-release matrix gradually liberates trospium into the bloodstream, sustaining effective concentrations and diminishing the risk of sudden peaks that may heighten side effects.
Available Strengths and Dosage Forms
Trofame XR is predominantly available in extended-release capsule form, facilitating once-daily dosing. Standardized strengths ensure precision in therapeutic outcomes.
Inactive Ingredients and Excipients
The formulation incorporates inert excipients that stabilize the capsule, optimize absorption, and safeguard the integrity of the active compound until ingestion.
Xanomeline-trospium
Xanomeline/trospium, also known by the brand name Cobenfy, is a medication used to treat schizophrenia in adults. It combines two drugs: xanomeline, a muscarinic agonist, and trospium chloride, a muscarinic antagonist. This combination is designed to improve tolerability compared to xanomeline alone.
Trospium vs oxybutynin
Oxybutynin is more lipophilic and can penetrate the central nervous system, which may lead to a higher incidence of cognitive side effects like confusion, dizziness, and hallucinations, particularly in older adults. Conversely, trospium is a quaternary amine, which makes it less able to cross the blood-brain barrier, resulting in fewer central nervous system side effects. Both drugs can cause common anticholinergic side effects such as dry mouth and constipation, but studies have shown trospium may cause less dry mouth.
Trospium vs Tolterodine
trospium is a quaternary amine, which prevents it from crossing the blood-brain barrier to a significant extent, thus minimizing CNS side effects like confusion and cognitive impairment, making it a safer option for elderly patients. In contrast, tolterodine is a tertiary amine that is more capable of penetrating the blood-brain barrier, increasing the risk of CNS-related adverse effects.
Trospium vs myrbetriq
rospium is an anticholinergic (antimuscarinic) agent that works by blocking muscarinic receptors on the bladder muscle to prevent involuntary contractions. Myrbetriq, on the other hand, is a beta-3 adrenergic agonist that directly activates beta-3 receptors in the bladder to relax the detrusor muscle and increase bladder capacity. This fundamental difference in action leads to different side effect profiles. While trospium's common side effects are characteristic of anticholinergic drugs, such as dry mouth and constipation, Myrbetriq's side effects can include elevated blood pressure, nasopharyngitis, and urinary tract infections.
Trospium Mechanism of Action
Antimuscarinic Activity on Bladder Smooth Muscle
Trospium binds competitively to muscarinic receptors located in detrusor smooth muscle. This blockade reduces parasympathetic stimulation, suppressing abnormal bladder contractions.
Reduction of Detrusor Overactivity and Urinary Urgency
By diminishing involuntary detrusor contractions, Trofame XR mitigates sudden urges and allows patients greater control over bladder function.
Pharmacokinetics: Absorption, Distribution, Metabolism, Excretion
- Absorption: Moderate oral bioavailability with improved control via extended-release delivery.
- Distribution: Widely dispersed, with minimal penetration into the central nervous system.
- Metabolism: Limited hepatic metabolism, primarily undergoing hydrolysis.
- Excretion: Predominantly eliminated unchanged through renal pathways.
Onset of Action and Duration of Therapeutic Effects
Clinical improvement is often observed within hours of initial dosing, with sustained benefits lasting up to 24 hours due to extended-release pharmacology.
Trospium Uses
Overactive Bladder (OAB) Treatment
The principal indication of Trofame XR is OAB, where it significantly decreases urgency, frequency, and urge incontinence episodes.
Management of Urinary Frequency and Urgency
It improves quality of life by stabilizing bladder function, reducing nocturnal awakenings, and enhancing social confidence.
Reduction of Urge Incontinence Episodes
Patients frequently report fewer incontinence accidents, translating into improved daily activities and reduced reliance on protective pads.
Use in Patients with Detrusor Overactivity Due to Neurological Conditions
Trofame XR is utilized in individuals with bladder hyperactivity associated with conditions such as multiple sclerosis and spinal cord injury, providing symptomatic relief.
Off-Label Uses of Trospium Chloride
Potential Role in Interstitial Cystitis and Bladder Pain Syndrome
Although not officially sanctioned, trospium has been investigated for alleviating discomfort and urgency in patients with interstitial cystitis.
Adjunctive Therapy for Neurogenic Bladder Dysfunction
In complex neurogenic bladder cases, trospium may be considered as part of a multifaceted treatment plan.
Investigational Use in Pediatric Urinary Incontinence
Preliminary studies explore trospium’s safety and efficacy in pediatric populations experiencing severe urinary incontinence.
Comparison with Other Antimuscarinic Therapies
Trospium is distinctive in its limited blood–brain barrier penetration, potentially offering reduced risk of central nervous system adverse events compared to other antimuscarinics.
Trospium chloride Dosage
Trospium Dosage
The typical regimen involves one extended-release capsule taken orally once daily.
Extended-Release Capsule Administration Guidelines
Capsules should be swallowed whole with water, without crushing or chewing, to preserve the controlled-release properties.
Timing of Doses and Considerations with Meals
Administration is recommended on an empty stomach, preferably one hour before meals, to optimize absorption.
Adjustments in Renal and Hepatic Impairment
Reduced dosing or careful monitoring may be necessary for patients with impaired kidney or liver function to avoid accumulation and toxicity.
Missed Dose and Overdose Management Guidance
If a dose is missed, it should be taken as soon as remembered unless it is near the next scheduled dose. Overdose requires immediate medical attention, with supportive interventions and monitoring.
Side Effects of Trofame XR
Trospium Chloride Side Effects
Adverse events typically stem from systemic anticholinergic activity, influencing gastrointestinal, ocular, and urinary systems.
Frequency and Severity of Reported Adverse Events
Most side effects are mild to moderate, with severe reactions being relatively uncommon.
Common Side Effects
- Dry mouth and throat irritation
- Constipation and abdominal discomfort
- Blurred vision and difficulty with ocular accommodation
- Headache and lightheadedness
- Urinary retention
Rare but Serious Side Effects
- Severe hypersensitivity or allergic reactions
- Confusion, hallucinations, or cognitive impairment
- Cardiac arrhythmias including tachycardia
Trospium side effects elderly
constipation, dry mouth, indigestion, stomach pain, or trouble passing urine)
Drug Interactions
Interactions with Other Anticholinergic Medications
Concurrent use with other antimuscarinics may intensify side effects such as dry mouth, constipation, or blurred vision.
Potential Interactions with CNS Depressants
Combined use with sedatives may exacerbate drowsiness, dizziness, or cognitive slowing.
Effects with Medications Metabolized by Renal Excretion Pathways
Since trospium is primarily renally excreted, caution is required when co-administered with drugs competing for renal clearance.
Interaction with Alcohol and Food Considerations
Alcohol may amplify central side effects, while food can reduce absorption efficiency, particularly with high-fat meals.
Trospium Contraindications
Hypersensitivity to Trospium Chloride or Formulation Components
Patients with known hypersensitivity reactions should avoid the drug entirely.
Urinary Retention or Gastric Retention
Individuals predisposed to retention syndromes may experience worsening of their condition.
Uncontrolled Narrow-Angle Glaucoma
Trospium may elevate intraocular pressure, making it unsuitable for patients with uncontrolled narrow-angle glaucoma.
Severe Renal or Hepatic Impairment
Significant organ dysfunction may alter drug clearance, raising toxicity risks and rendering use contraindicated.
Warnings and Precautions
Risk of Cognitive Side Effects in Elderly Patients
Elderly individuals are particularly vulnerable to central nervous system disturbances when exposed to antimuscarinic medications. Manifestations may include confusion, short-term memory lapses, and impaired concentration. These effects necessitate vigilant observation, especially during the initiation phase of therapy.
Increased Sensitivity in Patients with Autonomic Neuropathy
Patients with autonomic neuropathy may exhibit heightened sensitivity to trospium chloride. The altered neural control of bladder and gastrointestinal function can exacerbate drug-induced side effects, requiring meticulous dose titration.
Considerations for Patients with Gastrointestinal Obstruction
Trospium chloride may worsen gastrointestinal motility issues. In individuals with partial obstruction or reduced gastric emptying, the drug can precipitate severe constipation or ileus. Such cases demand careful benefit-risk assessment.
Caution in Patients with Cardiovascular Conditions
Patients with pre-existing cardiovascular disorders should use trospium cautiously. Tachyarrhythmias, palpitations, and elevated blood pressure have been reported in sensitive individuals. Clinical monitoring of cardiac function is strongly advised in these patients.
Careful Administration and Special Considerations
Dose Adjustment in Renal Insufficiency
As trospium is predominantly eliminated unchanged via renal pathways, impaired kidney function significantly alters systemic drug concentrations. Dose reduction or extended dosing intervals may be warranted to mitigate toxicity.
Monitoring in Hepatic Impairment
Although hepatic metabolism is limited, compromised liver function can still influence drug handling. Close supervision, along with liver function monitoring, is recommended in patients with hepatic insufficiency.
Clinical Vigilance in Patients with Comorbidities
Patients with multiple chronic diseases require individualized treatment plans. Conditions such as diabetes, hypertension, or neurological disorders may interact with trospium’s pharmacological profile, necessitating cautious oversight.
Trospium Alternatives
oxybutynin, tolterodine, darifenacin, and solifenacin, as well as the beta-3 adrenergic agonist mirabegron.
Administration in Special Populations
Administration to Elderly Patients
Increased Susceptibility to CNS Effects
Older adults are predisposed to dizziness, sedation, and disorientation when taking antimuscarinic drugs. This heightened susceptibility can increase fall risk and impair independence.
Cognitive and Anticholinergic Risk Considerations
Long-term anticholinergic therapy in elderly patients is associated with a greater probability of cognitive decline. A thorough cognitive assessment prior to initiation is advisable.
Lower Dose Recommendations and Monitoring
Initiating treatment with the lowest effective dose reduces adverse reactions. Regular re-evaluation of therapeutic benefit and side effect profile is essential.
Administration to Pregnant Women and Nursing Mothers
Safety Data in Pregnancy and Animal Studies
Animal studies have demonstrated some embryotoxic effects at high doses. Human data remain insufficient to establish definitive safety during pregnancy.
Risk–Benefit Evaluation During Gestation
Treatment in pregnant women should only proceed if the expected maternal benefit outweighs potential risks to the fetus. Non-pharmacological interventions may be preferred as first-line options.
Excretion into Breast Milk and Breastfeeding Guidance
Trospium chloride may be excreted in breast milk. Nursing mothers should avoid therapy or suspend breastfeeding during treatment to prevent exposure to the infant.
Administration to Children
Limited Evidence on Pediatric Safety and Efficacy
Clinical studies on pediatric populations are scarce, leaving the safety and effectiveness of trospium in children largely undetermined.
Current Recommendations and Restrictions
Routine use in children is not recommended due to insufficient evidence. Any administration should occur only under specialized medical supervision.
Potential Research Directions
Ongoing research is exploring the role of trospium in pediatric urinary incontinence. Future data may expand therapeutic indications for younger age groups.
Overdosage
Clinical Presentation of Trospium Overdose
Overdose may present with severe anticholinergic manifestations, often resembling delirium. Symptoms can be unpredictable and potentially life-threatening if not addressed rapidly.
Symptoms: Agitation, Hallucinations, Severe Dry Mouth, Urinary Retention
- Intense restlessness and agitation
- Visual or auditory hallucinations
- Pronounced xerostomia and difficulty swallowing
- Inability to void urine despite urge
Emergency Management: Gastric Lavage, Activated Charcoal, Supportive Care
Initial treatment includes gastric lavage if ingestion is recent. Activated charcoal may limit further absorption. Supportive therapy, including intravenous fluids and monitoring, forms the backbone of management.
Monitoring and Treatment in Hospital Settings
Continuous cardiac monitoring, respiratory support, and neurological evaluation are necessary in cases of severe overdose. Symptomatic treatment remains the primary approach.
Storage and Handling Precautions
Recommended Storage Conditions for Trofame XR
Store capsules at controlled room temperature, ideally between 20°C and 25°C. Avoid excessive fluctuations in environmental conditions.
Protection from Moisture, Heat, and Light
Exposure to humidity, heat, or direct sunlight can compromise capsule integrity. Medications should be kept in their original packaging until use.
Safe Handling Instructions to Avoid Accidental Exposure
Capsules must be swallowed whole. Breaking or crushing the formulation compromises the extended-release system and may increase side effects.
Expiry and Disposal Guidelines
Expired medication should not be consumed. Proper disposal through pharmacy take-back programs or as per local regulations is strongly encouraged to prevent environmental contamination.
Trofame XR, Trospium Chloride FAQ
- What is Trofame XR used for?
- What is trospium chloride used to treat?
- What is the best time to take trospium chloride?
- Is trospium hard on your kidneys?
- Can I drink coffee with trospium?
- How soon does trospium work?
- What happens if I take trospium with food?
- What is the side effect of trospium?
- Can trospium cause memory loss?
- Which is better, oxybutynin or trospium?
- Can I take trospium at bedtime?
- Does trospium increase heart rate?
- Is trospium well tolerated?
- What are the most common side effects of trospium?
- Can you drink coffee with trospium?
- Can trospium cause urinary retention?
- Can trospium make you sleepy?
- When should I stop taking trospium?
- Is trospium linked to dementia?
- What is Trofame XR used for?
- Can trospium cause memory loss?
- Can I take trospium at bedtime?
- Does trospium increase heart rate?
- Is trospium well tolerated?
- Is trospium linked to dementia?
What is Trofame XR used for?
The Trofame XR Capsule is a treatment for people who have an overactive bladder.
What is trospium chloride used to treat?
Trospium is used to treat the symptoms of an overactive bladder.
What is the best time to take trospium chloride?
Tablet is taken twice a day or once a day at bedtime.
Is trospium hard on your kidneys?
No
Can I drink coffee with trospium?
Yes, but limit intake.
How soon does trospium work?
Week 1
What happens if I take trospium with food?
Eating can interfere with how your body absorbs trospium. If you take it with food, your body might not absorb as much of it as it would on an empty stomach.
What is the side effect of trospium?
- Dizziness
- Drowsiness
- Blurred vision
- Confusion
Can trospium cause memory loss?
No
Which is better, oxybutynin or trospium?
Trospium
Can I take trospium at bedtime?
Yes
Does trospium increase heart rate?
Yes
Is trospium well tolerated?
Yes
What are the most common side effects of trospium?
- Back pain
- Blurred vision
- Constipation
- Dry mouth
- Dry skin
- Sleepiness
Can you drink coffee with trospium?
No
Can trospium cause urinary retention?
If you're taking trospium, you might find it tougher to go to the bathroom.
Can trospium make you sleepy?
Yes
When should I stop taking trospium?
If your overactive bladder symptoms are improving with trospium and you're not experiencing any issues, there's no reason to stop taking it.
Is trospium linked to dementia?
No
What is Trofame XR used for?
Trofame XR Capsule is actually used to treat an overactive bladder. Its designed to help with symptoms, like urination, leakage and loss of control.
Can trospium cause memory loss?
No
Can I take trospium at bedtime?
Yes
Does trospium increase heart rate?
Yes
Is trospium well tolerated?
Yes
Is trospium linked to dementia?
No