Introduction
Ceff, also known as Cephalexin Tablet, is a widely prescribed antibiotic belonging to the cephalosporin family. It is primarily used to combat a broad spectrum of bacterial infections. As a first-generation cephalosporin, it plays a vital role in eradicating susceptible bacteria and preventing the spread of infection.
Overview of Ceff (Cephalexin Tablet)
Cephalexin is valued for its broad coverage against common bacterial pathogens. It is considered safe, effective, and widely used in both outpatient and inpatient settings.
Classification: First-Generation Cephalosporin Antibiotic
This drug falls under the class of first-generation cephalosporins, which are chemically related to penicillins but have distinctive structural properties that enhance their therapeutic profile.
General Therapeutic Role in Bacterial Infections
Ceff is employed in managing infections of the respiratory tract, urinary tract, skin, bones, and ears. Its ability to inhibit bacterial growth makes it a frontline choice in many treatment regimens.
Composition
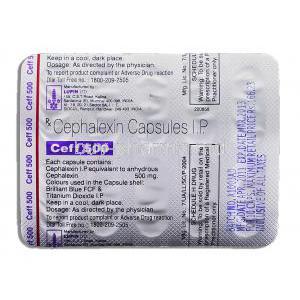
- Active ingredient: Cephalexin monohydrate
- Available strengths: Commonly supplied in 250 mg, 500 mg, and 1 g tablets or capsules
- Inactive ingredients: May include magnesium stearate, microcrystalline cellulose, and colorants, which stabilize the formulation
Mechanism of Action: How Cephalexin Works
Cephalexin exerts its antimicrobial action by targeting bacterial cell walls. This action disrupts bacterial survival and replication.
- Inhibition of cell wall synthesis: Prevents cross-linking of peptidoglycan, a structural component essential for bacterial survival
- Broad activity: Effective against Gram-positive bacteria such as Staphylococcus aureus and Gram-negative organisms like Escherichia coli
- Bactericidal effects: Leads to direct bacterial destruction rather than mere inhibition
- Resistance considerations: Some strains may develop beta-lactamase-mediated resistance, reducing effectiveness
Approved Medical Uses
- Respiratory tract infections including pharyngitis, tonsillitis, bronchitis, and pneumonia
- Skin and soft tissue infections such as cellulitis, abscesses, and wound infections
- Urinary tract infections including cystitis and pyelonephritis
- Bone and joint infections like osteomyelitis
- Otitis media and sinusitis
Off-Label Uses
- Prevention of recurrent urinary tract infections
- Treatment of bacterial endocarditis in patients allergic to penicillin
- Dental infection prophylaxis prior to dental procedures
- Alternative therapy for Lyme disease in pediatric or pregnant populations
Dosage and Administration
- Adults: Typically 250 mg to 500 mg every 6 to 12 hours, depending on severity
- Pediatrics: Dosage calculated based on weight, often 25–50 mg/kg/day divided into multiple doses
- Renal impairment: Adjustments required to prevent accumulation and toxicity
- Frequency: Usually administered 2–4 times daily for 7–14 days
- Missed dose: Should be taken as soon as remembered unless it is close to the next scheduled dose
- Administration: May be taken with or without food, though food may reduce gastric discomfort
Side Effects of Cephalexin
Common Side Effects
- Nausea, vomiting, and diarrhea
- Abdominal discomfort and indigestion
- Mild skin rashes or itching
- Dizziness and fatigue
Serious Side Effects
- Severe allergic reactions, including anaphylaxis
- Clostridioides difficile-associated diarrhea
- Elevated liver enzymes and hepatic impairment
- Hematologic changes such as eosinophilia and thrombocytopenia
- Seizures, particularly in patients with renal dysfunction receiving high doses
Warnings and Precautions
- Cross-reactivity: Patients allergic to penicillin may also exhibit hypersensitivity
- Antibiotic-associated colitis: Risk of severe diarrhea due to C. difficile infection
- Renal impairment: Dosage adjustment and monitoring required
- Superinfections: Prolonged therapy may allow resistant organisms or fungi to proliferate
- Driving precautions: Potential dizziness may impair the ability to operate vehicles or machinery safely
Contraindications
Ceff (Cephalexin) should not be administered in certain medical conditions due to the risk of severe adverse effects or treatment failure.
- Known hypersensitivity: Patients with documented allergies to cephalosporins or penicillins should avoid cephalexin as cross-reactivity may provoke severe reactions.
- History of severe allergic reactions: Individuals with prior episodes of anaphylaxis, Stevens-Johnson syndrome, or angioedema linked to beta-lactam antibiotics should not receive this therapy.
Drug Interactions
Cephalexin may interact with several pharmacological agents, altering efficacy or safety. Vigilant monitoring is recommended when co-administered with the following:
- Metformin: Concomitant use may increase serum metformin concentrations, heightening the risk of lactic acidosis.
- Oral contraceptives: Cephalexin may potentially reduce the effectiveness of hormonal birth control, necessitating additional contraceptive measures.
- Anticoagulants (Warfarin): Use with warfarin may prolong prothrombin time, increasing the risk of bleeding complications.
- Probenecid: Co-administration decreases renal clearance of cephalexin, raising plasma concentrations and prolonging its half-life.
- Nephrotoxic drugs: Concurrent use with aminoglycosides, loop diuretics, or other nephrotoxic agents may potentiate renal injury.
Administration in Special Populations
Elderly Patients
Aging affects drug absorption, distribution, and elimination. Cephalexin dosing must be tailored carefully in older adults.
- Pharmacokinetic changes: Reduced renal clearance may lead to drug accumulation and heightened risk of adverse effects.
- Renal function adjustments: Dose reductions or extended dosing intervals are often necessary to prevent toxicity.
- Monitoring: Regular assessment for gastrointestinal intolerance, dizziness, or skin reactions is crucial.
Pregnant Women and Nursing Mothers
The use of cephalexin during pregnancy and breastfeeding requires careful evaluation of benefits versus potential risks.
- Pregnancy safety: Classified as generally safe, cephalexin crosses the placenta but has not been shown to cause teratogenic effects in humans.
- Breast milk excretion: Small amounts are excreted into breast milk, which may occasionally cause diarrhea or candidiasis in nursing infants.
- Risk-benefit assessment: Treatment should only proceed when the therapeutic advantages outweigh potential neonatal exposure risks.
Children and Infants
Pediatric use of cephalexin is common, but dosing precision is paramount.
- Safety profile: Generally well tolerated in children with an adverse event spectrum similar to adults.
- Dose calculation: Typically based on body weight, ranging between 25–50 mg/kg/day, divided into multiple doses.
- Neonatal limitations: Limited data in neonates due to immature renal function necessitates caution and adjusted dosing schedules.
Overdose and Emergency Management
An overdose of cephalexin, though rare, requires immediate medical attention.
- Symptoms: Common signs include nausea, vomiting, epigastric discomfort, and in severe cases, seizures.
- Supportive care: Management is symptomatic, focusing on rehydration and maintaining electrolyte balance.
- Decontamination: Gastric lavage and activated charcoal may be considered if ingestion is recent.
- Severe toxicity: Hemodialysis can be employed to enhance drug clearance in patients with impaired renal function or life-threatening overdose.
Storage and Handling Precautions
Proper storage and handling ensure that cephalexin maintains its stability and therapeutic effectiveness.
- Temperature: Store at controlled room temperature, ideally between 20–25°C.
- Moisture and light protection: Keep in a dry environment, away from direct sunlight and excessive humidity.
- Safe disposal: Unused or expired medication should be discarded according to local pharmaceutical disposal guidelines to prevent misuse.
- Shelf life: Refer to the manufacturer’s label for expiration dates, and do not consume the medication beyond its indicated validity.
Ceff, Cephalexin Tablet FAQ
- What are cephalexin tablets used for?
- Is cephalexin 500 mg 3 times a day?
- Why is cephalexin used for UTI?
- What should you avoid while taking cephalexin?
- Is cephalexin a strong antibiotic?
- Is cephalexin the same as amoxicillin?
- What painkillers can I take with cephalexin?
- Can I take cephalexin and paracetamol together?
- Does cephalexin make you tired?
- How long does cephalexin take to heal a UTI?
- Can cephalexin cause yeast infection?
- How quickly does cephalexin work?
- Can I drink coffee while taking cephalexin?
- Why can't you lie down after taking cephalexin?
- What is the most common side effect of cephalexin?
- Is cephalexin safe for kidneys?
- Does cephalexin affect sleep?
- How to know if cephalexin is effective?
- Who should not take cephalexin?
- Which antibiotic is similar to cephalexin?
- Which is stronger, cephalexin or azithromycin?
- How long does cephalexin stay in your system?
- Can I take vitamin D with cephalexin?
- Can I take Advil with cephalexin?
- Can cephalexin cause joint and muscle pain?
- Does cephalexin change your urine color?
- How to avoid side effects of cephalexin?
- How long does it take for cephalexin to work?
- Is cephalexin a powerful antibiotic?
- What foods should you avoid while taking cephalexin?
- Is 500 mg 4 times a day of cephalexin a lot?
- Which bacteria are resistant to cephalexin?
- What can you not take with cephalexin?
- Can cephalexin 500 mg treat tooth infection?
- What is cephalexin mainly used for?
- Can I take cephalexin and paracetamol together?
- Can I take Advil with cephalexin?
- Can cephalexin cause joint and muscle pain?
- Does cephalexin change your urine color?
- How long does it take for cephalexin to work?
- Is cephalexin a powerful antibiotic?
- Which is stronger, Ciprofloxacin or cephalexin?
- Can cephalexin 500 mg treat tooth infection?
What are cephalexin tablets used for?
Cephalexin is an antibiotic that's pretty effective against infections. It's often used to treat pneumonia and other respiratory infections. It also works for bone, skin, and ear infections as well as genital and urinary tract infections.
Is cephalexin 500 mg 3 times a day?
For Adults
Why is cephalexin used for UTI?
Cephalexin gets absorbed by the body easily and penetrates deep into the urinary tract.
What should you avoid while taking cephalexin?
Do not take cephalexin with zinc supplements.
Is cephalexin a strong antibiotic?
Cephalexin has excellent activity against gram-positive staphylococci and streptococci bacteria
Is cephalexin the same as amoxicillin?
Cephalexin and amoxicillin are two kinds of antibiotics. Cephalexin is a type of cephalosporin while amoxicillin belongs to the penicillin family.
What painkillers can I take with cephalexin?
acetaminophen and ibuprofen
Can I take cephalexin and paracetamol together?
Yes
Does cephalexin make you tired?
Yes
How long does cephalexin take to heal a UTI?
3 to 7 days
Can cephalexin cause yeast infection?
Yes
How quickly does cephalexin work?
2-3 days
Can I drink coffee while taking cephalexin?
Yes
Why can't you lie down after taking cephalexin?
When you're taking antibiotics try not to lie down. This is because it can slow down how quickly the medicine gets from your throat into your stomach.
What is the most common side effect of cephalexin?
- Nausea
- Vomiting
- Indigestion
- Diarrhea
Is cephalexin safe for kidneys?
If you've got kidney problems, be careful when taking cephalexin.
Does cephalexin affect sleep?
No
How to know if cephalexin is effective?
You will see improvement in a few days.
Who should not take cephalexin?
- Allergic reaction to Cephalexin
- Kidney problems
- Skin rash
- Mouth sores
Which antibiotic is similar to cephalexin?
Which is stronger, cephalexin or azithromycin?
Azithromycin
How long does cephalexin stay in your system?
8 hours
Can I take vitamin D with cephalexin?
Yes
Can I take Advil with cephalexin?
Yes
Can cephalexin cause joint and muscle pain?
Yes
Does cephalexin change your urine color?
Yes
How to avoid side effects of cephalexin?
Avoid spicy foods
How long does it take for cephalexin to work?
48 hours
Is cephalexin a powerful antibiotic?
Yes
What foods should you avoid while taking cephalexin?
Foods with zinc, calcium-rich foods, Alcohol
Is 500 mg 4 times a day of cephalexin a lot?
Usually, you'd take 250 500 mg of cephalexin four times a day.
Which bacteria are resistant to cephalexin?
Methicillin-resistant Staphylococcus aureus (MRSA), most Enterococcus, or Pseudomonas
What can you not take with cephalexin?
- Probenecid
- Metformin
- Diuretics
Can cephalexin 500 mg treat tooth infection?
Yes
What is cephalexin mainly used for?
Cephalexin is an antibiotic that can tackle bacterial infections all over the body. It's part of a group of medicines called cephalosporins.
Can I take cephalexin and paracetamol together?
Yes
Can I take Advil with cephalexin?
Yes
Can cephalexin cause joint and muscle pain?
Yes
Does cephalexin change your urine color?
Yes
How long does it take for cephalexin to work?
48 hours
Is cephalexin a powerful antibiotic?
Yes
Which is stronger, Ciprofloxacin or cephalexin?
Ciprofloxacin
Can cephalexin 500 mg treat tooth infection?
Yes