Introduction to Faslodex Depot Injection
Faslodex Depot Injection, containing the active pharmaceutical ingredient fulvestrant, is a potent therapy used primarily in the treatment of certain forms of advanced breast cancer. It belongs to a novel class of endocrine therapies known as selective estrogen receptor degraders (SERDs), which function by binding to and facilitating the degradation of estrogen receptors.
Designed as a long-acting intramuscular formulation, Faslodex offers sustained suppression of estrogen receptor activity, making it particularly suitable for patients with hormone receptor-positive (HR+), HER2-negative breast cancer. Approved for use in postmenopausal women, it is a valuable treatment option, especially when disease progression occurs following anti-estrogen therapies such as tamoxifen or aromatase inhibitors.
Faslodex received its initial regulatory approval in 2002 by the U.S. FDA and has since become a cornerstone therapy in hormone-sensitive breast cancer management across global oncology practices.
Composition and Formulation Details
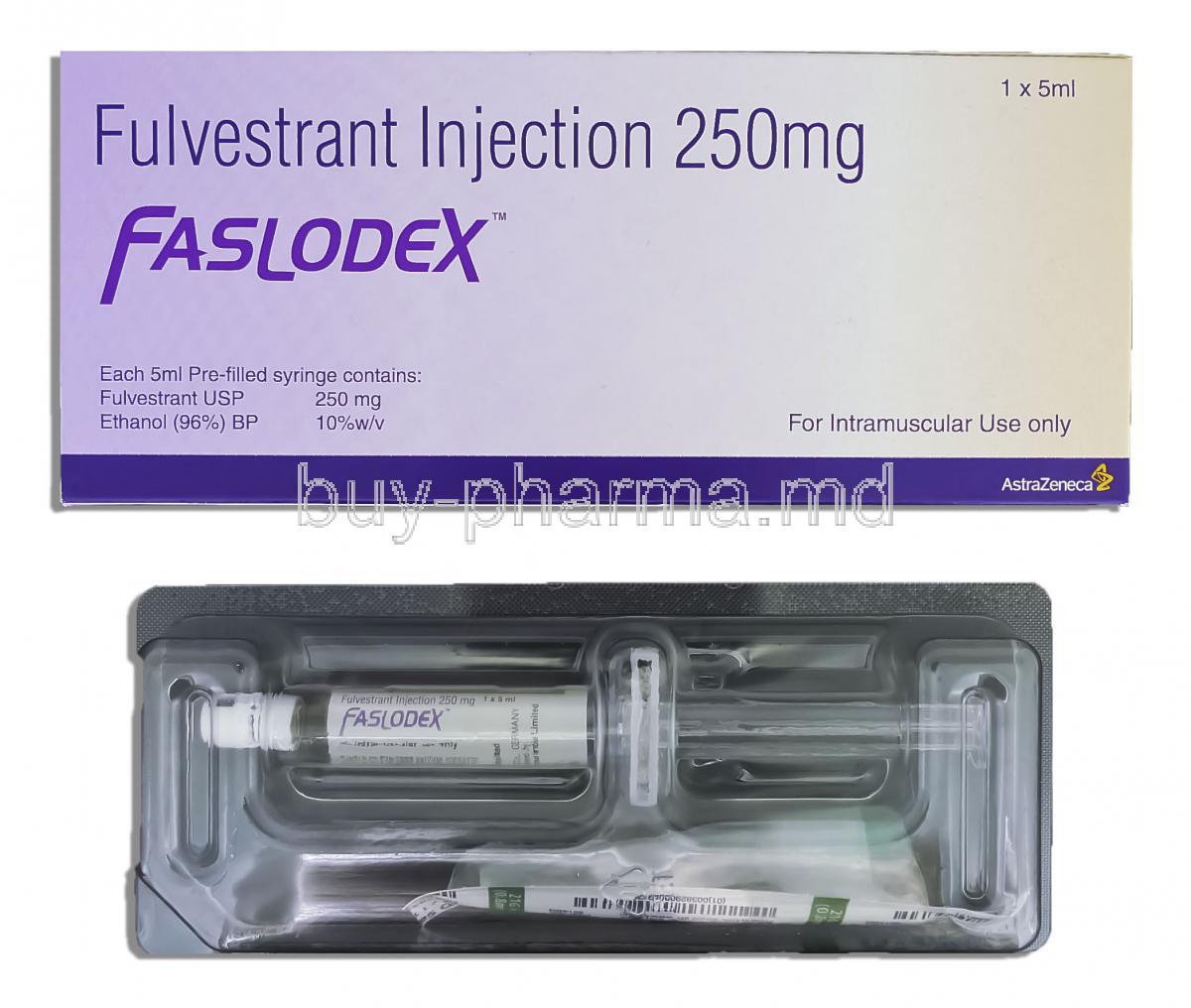
Each milliliter of Faslodex Depot Injection contains 250 mg of fulvestrant as the active ingredient. The high-concentration formulation enables effective monthly dosing, reducing the burden of frequent administration.
In addition to fulvestrant, the injection contains several pharmaceutical excipients and solvents, including ethanol, benzyl alcohol, benzyl benzoate, and castor oil. These components are essential for creating a stable, oil-based depot formulation that ensures gradual release and prolonged systemic exposure.
The product is presented as a clear, colorless to yellow, viscous solution in prefilled syringes or glass vials. It is typically supplied in 5 mL vials containing 250 mg of fulvestrant, either as a single unit or in packs containing multiple doses for clinical convenience.
Mechanism of Action: How Faslodex Works
Faslodex operates by competitively binding to estrogen receptors (ER) on tumor cells, acting as a high-affinity antagonist. Unlike selective estrogen receptor modulators (SERMs), fulvestrant not only blocks receptor activation but also induces receptor degradation via the proteasome pathway, effectively downregulating ER levels in cancer cells.
This dual-action mechanism leads to an interruption of estrogen-mediated transcriptional activity, ultimately suppressing cell proliferation in hormone receptor-positive malignancies.
- Estrogen receptor antagonism disrupts downstream signaling pathways crucial for tumor growth.
- Fulvestrant is particularly effective in tumors resistant to other endocrine therapies due to its irreversible receptor degradation.
- Compared to aromatase inhibitors that suppress systemic estrogen production, Faslodex directly targets the receptor, making it a strategic option in advanced-stage disease.
Pharmacokinetically, fulvestrant demonstrates slow and consistent absorption from the intramuscular depot, extensive distribution in fatty tissues, hepatic metabolism via CYP3A4, and elimination predominantly through fecal excretion. The half-life supports a once-monthly dosing regimen, with steady-state concentrations achieved after repeated administration.
Approved Medical Uses of Faslodex Depot Injection
Faslodex is indicated for the treatment of HR+, HER2-negative advanced or metastatic breast cancer in postmenopausal women. It is particularly effective in patients who have experienced disease progression following previous anti-estrogen therapy.
- As a monotherapy in endocrine-sensitive tumors after tamoxifen failure
- In combination with CDK4/6 inhibitors such as palbociclib or abemaciclib for enhanced progression-free survival
- As an option for patients intolerant to aromatase inhibitors
In clinical guidelines, Faslodex is often recommended for second-line endocrine therapy and has shown meaningful clinical benefit in delaying disease progression and improving quality of life.
Off-Label and Investigational Uses
Beyond its primary indication, Faslodex has shown promise in several off-label and investigational settings. These applications are under ongoing evaluation in clinical trials and specialist use cases:
- Male breast cancer: Hormone receptor-positive male breast cancer has been treated with fulvestrant in rare but documented cases.
- Uterine sarcomas: Endometrial stromal tumors and leiomyosarcomas expressing estrogen receptors have shown limited responses to SERD therapy.
- Hormone-refractory prostate cancer: As part of combined endocrine therapy in experimental models.
- Combination trials: Use with PI3K/mTOR pathway inhibitors and other targeted agents for synergistic tumor control.
While not yet approved for these uses, the mechanistic rationale and emerging evidence support further exploration of Faslodex across diverse hormone-driven malignancies.
Dosage and Administration Guidelines
Faslodex is administered via intramuscular injection, typically in the gluteal muscle. The dosing regimen includes:
- Loading dose: 500 mg on Days 0, 14, and 28
- Maintenance dose: 500 mg every 28 days thereafter
Injections are divided equally between the two buttocks to reduce local irritation. Each 250 mg dose is administered slowly into the muscle using aseptic technique.
For patients with mild to moderate hepatic impairment, dose reduction is not usually necessary, though caution is advised. No adjustments are required based on renal function. Patients should be monitored for tolerability and compliance.
If a dose is missed, it should be administered as soon as possible without waiting for the next scheduled cycle. Therapy should then resume according to the original schedule.
Safe Administration Practices and Monitoring
Before initiating Faslodex therapy, healthcare providers should perform a comprehensive clinical evaluation, including liver function tests and confirmation of hormone receptor status.
- Use sterile equipment and follow proper intramuscular injection protocols.
- Rotate injection sites to avoid local tissue injury or lipodystrophy.
- Observe patients for 15–30 minutes post-injection for signs of allergic reaction or localized adverse effects.
Regular monitoring should include liver enzyme panels, complete blood counts, and imaging to assess tumor response. Any persistent injection site pain, swelling, or systemic side effects should be promptly evaluated.
Side Effects and Adverse Reactions
8.1 Common Side Effects
While Faslodex is generally well-tolerated, patients frequently experience mild to moderate side effects, especially during the early phases of treatment. Most are self-limiting and manageable with symptomatic care or dose adjustments.
- Injection site reactions: Pain, swelling, or erythema may occur due to the viscous nature of the depot formulation.
- Gastrointestinal discomfort: Nausea is commonly reported, often without vomiting.
- Systemic effects: Fatigue, asthenia, and hot flashes reflect the drug’s estrogen-depleting action.
- Musculoskeletal pain: Arthralgia, back pain, and muscle stiffness may develop with prolonged use.
- Liver function abnormalities: Transient increases in hepatic transaminases (ALT, AST) can occur and should be monitored periodically.
8.2 Serious or Rare Adverse Effects
Though rare, Faslodex may be associated with more severe adverse events, necessitating immediate medical attention or discontinuation of therapy.
- Thromboembolic complications: Deep vein thrombosis and pulmonary embolism, though uncommon, have been reported, particularly in patients with a predisposition.
- Hypersensitivity: Anaphylaxis and angioedema are extremely rare but require emergency intervention.
- Genital bleeding: Vaginal bleeding and endometrial thickening have been observed, especially in patients transitioning from prior hormone therapies.
- Hepatotoxicity: Severe hepatic injury is rare but may necessitate discontinuation in patients with preexisting liver conditions.
Drug and Substance Interactions
Faslodex does not exhibit extensive pharmacological interactions but still warrants caution in select co-prescription scenarios.
- Estrogen-based therapies: Concurrent use negates therapeutic action and is contraindicated.
- CYP3A4 modulators: Fulvestrant is metabolized via CYP3A4; inducers (e.g., rifampin, carbamazepine) may lower its efficacy, while inhibitors (e.g., ketoconazole) could enhance toxicity.
- Anticoagulants and antiarrhythmics: Risk of bleeding or altered metabolism should be carefully assessed.
- Food and alcohol: No clinically significant interactions have been documented.
Contraindications for Use
Faslodex is contraindicated in specific populations and clinical conditions where its use may pose unacceptable risk.
- Known hypersensitivity: History of allergic reactions to fulvestrant or formulation excipients.
- Severe hepatic dysfunction: Metabolism may be impaired, increasing systemic exposure and toxicity.
- Pregnancy and lactation: Faslodex may cause fetal harm and is contraindicated in pregnant or breastfeeding women.
- Bleeding disorders: Intramuscular administration poses a risk of hematoma in patients on anticoagulation therapy or with coagulopathies.
Important Warnings and Precautionary Statements
Before initiating Faslodex, clinicians must consider several safety issues to ensure optimal patient outcomes and minimize adverse events.
- Hepatic monitoring: Chronic administration may lead to cumulative liver toxicity; liver enzymes should be periodically assessed.
- Bone mineral density: Long-term estrogen suppression can reduce bone density; DEXA scans may be advised in high-risk individuals.
- Injection-related bleeding: Caution should be exercised in patients receiving anticoagulants to avoid intramuscular hematoma formation.
- Effective contraception: Due to teratogenic potential, non-hormonal contraceptive methods are required during treatment and for several months post-therapy.
Special Population Administration Guidelines
12.1 Use in Elderly Patients
In elderly individuals, age-related physiological changes necessitate individualized risk-benefit assessments.
- Pharmacodynamics: Sensitivity to fulvestrant may be altered; lower muscle mass may affect IM administration comfort.
- Polypharmacy: Increased risk of drug-drug interactions and adverse reactions must be accounted for.
- Monitoring needs: Close surveillance of hepatic function, renal status, and mobility (due to musculoskeletal side effects) is essential.
12.2 Use During Pregnancy and Breastfeeding
Faslodex is strictly contraindicated during pregnancy and lactation due to fetal toxicity concerns observed in preclinical studies.
- Animal studies: Embryotoxicity, fetal malformations, and pregnancy loss have been reported in multiple species.
- Pregnancy category: Categorized as Category D (USA), indicating evidence of human fetal risk.
- Breastfeeding considerations: Unknown if fulvestrant is excreted in human milk; due to potential harm, breastfeeding is not recommended during therapy.
- Counseling: Women of reproductive age should be informed of these risks and use effective contraception.
12.3 Pediatric Use and Safety
Faslodex is not approved for pediatric use, and safety and efficacy have not been established in this population.
- Lack of data: No robust clinical trials conducted in children to date.
- Investigational use: Experimental protocols exist in pediatric oncology for hormone-sensitive tumors, though use remains off-label.
- Ethical oversight: Clinical trials involving children must be stringently monitored and approved by ethics boards.
Overdose and Emergency Management
Although rare, overdose with Faslodex may occur due to dosing errors or improper administration.
- Symptoms: Potential signs include nausea, fatigue, dizziness, and elevations in hepatic enzymes.
- Initial management: Supportive care is the primary intervention, as no specific antidote exists.
- Monitoring: Evaluate liver and renal function, and maintain hemodynamic stability in acute scenarios.
- Reporting: All suspected overdose cases should be documented and reported to pharmacovigilance authorities.
Storage and Shelf Life Guidelines
Proper storage of Faslodex is crucial to maintain efficacy and safety.
- Temperature control: Store at 2°C–8°C (36°F–46°F). Do not freeze.
- Pre-administration care: Allow vial to reach room temperature before administration to ease injection discomfort.
- Post-opening stability: Use immediately once opened; do not store partially used syringes.
- Expiry tracking: Regularly inspect packaging for expiry date, integrity, and signs of contamination.
Handling Precautions for Healthcare Providers and Caregivers
Due to its cytotoxic profile, Faslodex should be handled with care by trained personnel.
- PPE: Use gloves, gowns, and eye protection to minimize exposure risk.
- Needle disposal: Dispose of used syringes in puncture-resistant sharps containers.
- Waste management: Comply with institutional and governmental regulations for hazardous medical waste disposal.
- Spill protocol: Clean spills using cytotoxic spill kits; avoid skin and mucosal contact.
Patient Counseling and Educational Guidance
Patient education plays a vital role in successful therapy outcomes and safety adherence.
- Dosing schedule: Stress the importance of adhering to injection appointments to maintain therapeutic levels.
- Side effect management: Teach patients to identify and report any signs of severe reaction, such as shortness of breath or unusual bleeding.
- Drug communication: Encourage patients to inform their care team of all medications and supplements taken.
- Support resources: Offer access to patient information leaflets, helplines, and counseling services to address emotional and treatment-related concerns.