Insulatard Flexpen Pen-filled Injection
- Introduction to Insulatard FlexPen Pen-filled Injection
- Composition and Formulation of Insulatard FlexPen
- Mechanism of Action: How Insulatard FlexPen Works
- Indications and Approved Uses of Insulatard FlexPen
- Off-Label Uses of Insulatard FlexPen
- Dosage and Administration Guidelines for Insulatard FlexPen
- Important Storage Instructions for Insulatard FlexPen
- Common Side Effects of Insulatard FlexPen
- Other Possible Side Effects and Adverse Reactions
- Warnings and Precautions for Insulatard FlexPen Use
- Contraindications to Using Insulatard FlexPen
- Drug and Substance Interactions with Insulatard FlexPen
- Careful Administration of Insulatard FlexPen
- Administration of Insulatard FlexPen to Elderly Patients
- Administration of Insulatard FlexPen in Pregnant and Nursing Women
- Administration of Insulatard FlexPen in Pediatric Populations
- Overdose and Emergency Management with Insulatard FlexPen
- Handling Precautions and Safe Disposal of Insulatard FlexPen
Introduction to Insulatard FlexPen Pen-filled Injection
Overview of Insulatard FlexPen and Its Therapeutic Classification
Insulatard FlexPen is a prefilled insulin delivery device classified as an intermediate-acting insulin. It contains isophane insulin, also known as NPH (Neutral Protamine Hagedorn) insulin, designed for basal glycemic control in individuals with diabetes mellitus.
Historical Development and Approval Milestones
Introduced by Novo Nordisk, Insulatard FlexPen gained regulatory approval following comprehensive clinical trials demonstrating its efficacy and safety. Its user-friendly design revolutionized insulin therapy by offering enhanced dosing accuracy and convenience.
Purpose and Benefits of Premixed Pen-filled Insulin Devices
- Facilitates precise insulin administration
- Minimizes preparation errors
- Enhances portability and ease of use for active lifestyles
- Reduces the risk of dosage miscalculations
Composition and Formulation of Insulatard FlexPen
Active Ingredient: Isophane Insulin (NPH Insulin)
The main element known as isophane insulin offers a long-lasting reduction in glucose levels that's ideal for meeting the basic insulin needs.
Inactive Ingredients and Their Roles
- Zinc chloride- Stabilizes insulin hexamers
- Protamine sulfate- Prolongs insulin action
- Phenol and m-cresol- Preserve sterility and stability
- Sodium chloride- Adjusts isotonicity
- Hydrochloric acid/sodium hydroxide- pH adjustment
- Water for injection- Solvent medium
Pharmaceutical Characteristics: Appearance, Concentration, and Packaging
Insulatard FlexPen is a cloudy, white suspension available at a concentration of 100 units/mL, packaged in a 3 mL disposable cartridge integrated within the pen device.
Mechanism of Action: How Insulatard FlexPen Works
Pharmacodynamics of Isophane Insulin
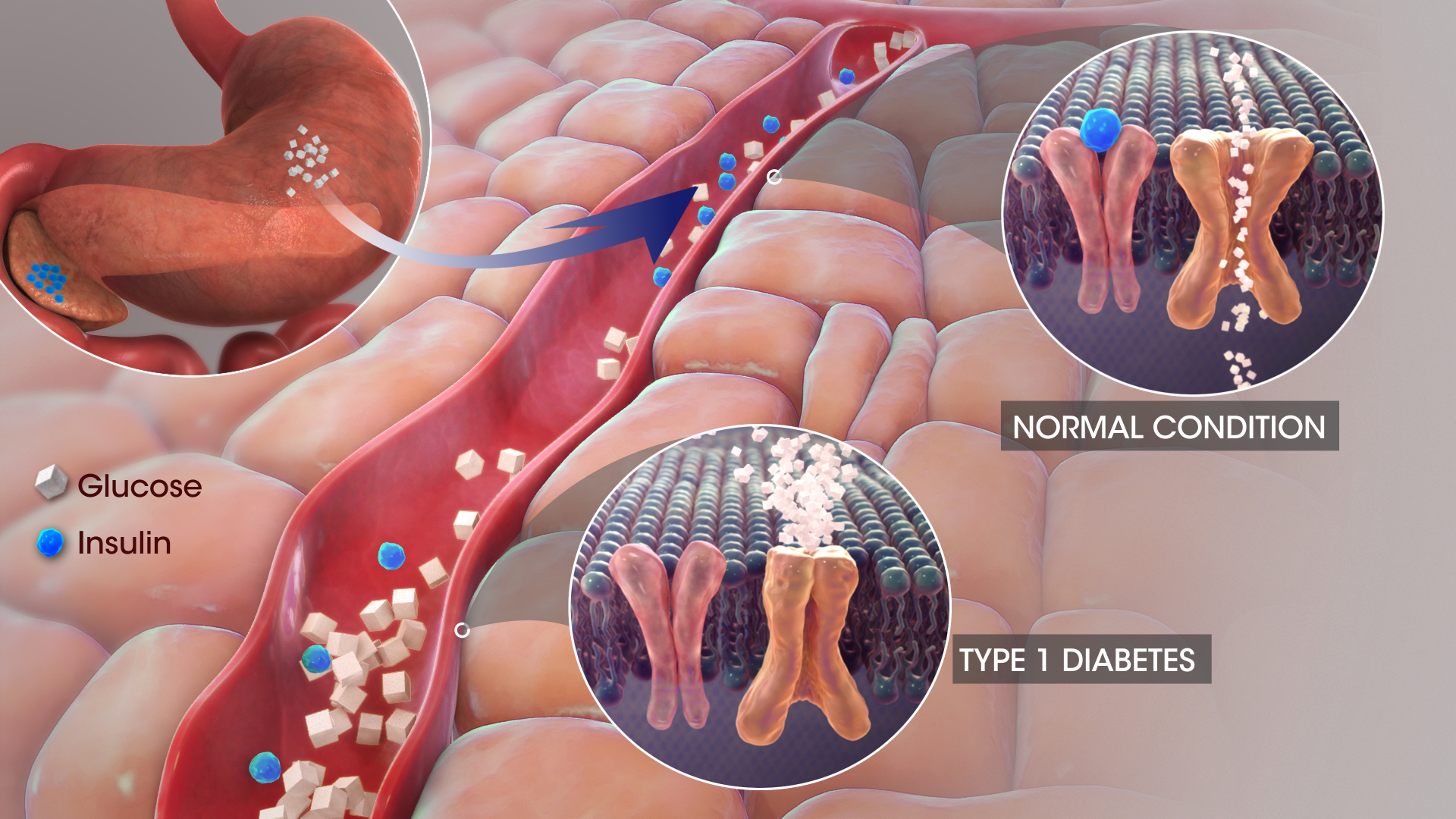
Isophane insulin exhibits a two-phase absorption pattern due to the inclusion of protamine, which delays its onset and prolongs its effectiveness in lowering glucose levels.

Onset, Peak, and Duration of Action
- Onset: Approximately 1.5 to 2 hours post-injection
- Peak effect: 4 to 12 hours
- Duration: Up to 24 hours
Role of Intermediate-acting Insulin in Glucose Regulation
Intermediate-acting insulins, such as Insulatard, maintain baseline insulin levels between meals and overnight, complementing the action of mealtime (prandial) insulins when needed.
Indications and Approved Uses of Insulatard FlexPen
Management of Type 1 Diabetes Mellitus
Management of Type 2 Diabetes Mellitus
Use in Combination with Rapid-acting Insulins or Oral Antidiabetic Agents
Insulatard can be taken together with working insulins or medications, like metformin and sulfonylureas to improve blood sugar management.
Off-Label Uses of Insulatard FlexPen
Use in Gestational Diabetes Management

Use in Hospitalized Patients with Hyperglycemia
Utilized in acute care settings to manage stress-induced hyperglycemia, maintaining glycemic stability during hospitalization.
Pediatric Diabetes Care Beyond Standard Approvals
Although formally approved for adults, Insulatard is sometimes used in pediatric populations under specialist supervision for individualized diabetes management.
Dosage and Administration Guidelines for Insulatard FlexPen
General Dosing Recommendations
Dosing is individualized based on metabolic needs, blood glucose monitoring, and physician guidance, typically ranging from 0.3 to 1.0 units/kg/day.
Initial Dosing for Insulin Patients
Initial dosages generally start low, often at 10 units once or twice daily, with subsequent titration based on self-monitored blood glucose profiles.
Dose Adjustment Based on Blood Glucose Monitoring
Regularly checking is vital for making changes to the insulin dosage, which depends upon the blood sugar levels before and after meals to optimize insulin treatment.
Step-by-Step Guide to Using the FlexPen Device
- Wash hands thoroughly before use
- Gently roll the pen between the palms to resuspend insulin
- Attach a new needle and prime the pen with two units
- Dial the prescribed dose
- Insert the needle into the subcutaneous tissue (abdomen or thigh preferred)
- Inject slowly and leave the needle in place for 6 seconds
- Remove the needle and dispose of it safely
Importance of Injection Timing Relative to Meals
Administer Insulatard approximately the same time each day. If combined with bolus insulin, coordinate injection timing carefully to balance postprandial glucose excursions.
Important Storage Instructions for Insulatard FlexPen
Proper Refrigeration and Room Temperature Storage
It is recommended to store Flexpens in a refrigerator (between 36°F and 46°F). Once opened and used for the intended time, they can be stored at room temperature (below 86°F) for a maximum of 6 weeks.
Shelf-Life Before and After Opening
Before opening, Insulatard FlexPen retains efficacy until the expiration date. After opening, it should be discarded after 42 days, even if insulin remains.
Protection from Light and Heat
Always keep the pen cap on to shield from direct light and avoid freezing, as these conditions compromise insulin potency.
Common Side Effects of Insulatard FlexPen
Hypoglycemia: Symptoms and Management
- Common symptoms: tremors, sweating, palpitations, confusion
- Management: ingestion of quick-acting carbohydrates such as glucose tablets or juice
Local Injection Site Reactions
Redness, swelling, or itching at the injection site may occur, usually resolving spontaneously without medical intervention.
Weight Gain Considerations
Lipodystrophy and Skin Changes at Injection Sites
Frequent injections at the same site can lead to lipoatrophy (fat loss) or lipohypertrophy (fat accumulation), emphasizing the importance of site rotation.
Other Possible Side Effects and Adverse Reactions
Allergic Reactions: Mild to Severe
Local hypersensitivity could show up as a rash or redness on the skin, while systemic reactions are uncommon but need care.
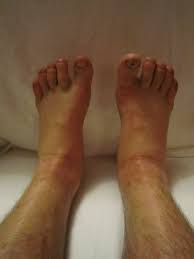
Sodium Retention and Edema
Rare Hypersensitivity Responses
In situations, there might be instances of severe allergic reactions that could require immediate discontinuation of treatment and proper emergency care.
Warnings and Precautions for Insulatard FlexPen Use
Risk of Hypoglycemia and Hyperglycemia
Insulatard FlexPen carries inherent risks of both hypoglycemia and hyperglycemia. Hypoglycemia, the most common adverse effect, may result from excessive dosing, skipped meals, or unanticipated physical exertion. Conversely, insufficient dosing or missed injections can precipitate hyperglycemia, elevating long-term risks of microvascular and macrovascular complications.

Importance of Self-Monitoring Blood Glucose (SMBG)
Regular self-monitoring of blood glucose is crucial for accurately titrating insulin doses and detecting asymptomatic glycemic excursions. SMBG empowers patients to make informed adjustments in diet, physical activity, and insulin administration, thereby optimizing therapeutic outcomes.
Precautions During Infections, Surgery, or Stress
Acute illnesses, surgical interventions, and physiological stressors can induce insulin resistance, necessitating vigilant monitoring and potential insulin dose adjustments. Patients should consult healthcare providers promptly to adjust therapy during such periods.
Contraindications to Using Insulatard FlexPen
Hypersensitivity to Isophane Insulin or Any Excipients
Individuals exhibiting hypersensitivity reactions to isophane insulin or formulation excipients must not use Insulatard FlexPen. Severe allergic responses may necessitate immediate discontinuation and alternative insulin therapies.
Episodes of Severe Hypoglycemia
Patients with a history of recurrent or unawareness-associated severe hypoglycemia are contraindicated for Insulatard FlexPen use unless adequate measures are instituted to mitigate recurrence.
Drug and Substance Interactions with Insulatard FlexPen
Interaction with Oral Antidiabetic Medications
Concurrent use with oral hypoglycemic agents, such as sulfonylureas or meglitinides, may increase the risk of hypoglycemia, requiring careful dose adjustment and increased monitoring.
Effects of Corticosteroids, Thyroid Hormones, and Sympathomimetics
- Corticosteroids may induce hyperglycemia by promoting gluconeogenesis and reducing insulin sensitivity.
- Thyroid hormones can accelerate insulin metabolism, potentially necessitating dose adjustments.
- Sympathomimetics, including beta-agonists, may alter glucose regulation, requiring clinical vigilance.
Alcohol and Beta-Blocker Interaction Risks
Alcohol consumption can unpredictably potentiate or mask hypoglycemic symptoms. Beta-blockers may obscure adrenergic manifestations of hypoglycemia, delaying recognition and treatment of low blood sugar events.
Careful Administration of Insulatard FlexPen
Rotation of Injection Sites to Prevent Lipodystrophy
To maintain insulin absorption and effectiveness, it's essential to rotate between authorized injection sites, such as the abdomen, thighs, buttocks, and upper arms, to prevent a condition called lipodystrophy.
Ensuring Proper Resuspension Before Injection
Gently roll the Insulatard FlexPen between your palms for approximately 10 times. Invert it at least 10 times to make sure the insulin is evenly distributed.
Avoiding Dosage Errors with Different Insulin Types
Patients must be careful to differentiate Insulatard from types of insulin— fast-action analogues—to avoid serious dosage mistakes that could have severe consequences.
Administration of Insulatard FlexPen to Elderly Patients
Adjustments Based on Renal or Hepatic Function Decline
As people get older, their kidney or liver functions may decrease, which can affect how the body processes insulin, leading to the need for adjustment of dosage and frequent monitoring to avoid low blood sugar levels.
Increased Risk of Hypoglycemia in Aging Populations
Older individuals could have reduced sensitivity to blood sugar levels. May benefit from regular monitoring of glucose levels and educational support for caregivers as essential aspects of their care routine.
Monitoring Strategies for Optimal Glycemic Control
Regularly checking HBA 11C levels and following a self-monitoring blood glucose routine can help in maintaining blood sugar goals and reducing any potential negative impacts on health.
Administration of Insulatard FlexPen in Pregnant and Nursing Women
Insulin Requirements During Pregnancy and Postpartum
Insulin needs typically escalate during pregnancy and decline postpartum. Close dose adjustments and fetal monitoring are vital to avert maternal and neonatal complications.
Safety Considerations for Breastfeeding Mothers
Insulatard is generally considered safe during lactation, but vigilance is warranted to monitor maternal glycemic fluctuations that could impact breastfeeding dynamics.

Fetal and Neonatal Monitoring Protocols
Regular ultrasound assessments and neonatal glucose evaluations post-delivery are recommended to ensure optimal maternal-fetal outcomes during insulin therapy.
Administration of Insulatard FlexPen in Pediatric Populations
Pediatric Dosing Guidelines
Dosing regimens must be individualized based on weight, growth patterns, and daily activity levels, with frequent reassessments as the child matures.
Special Monitoring in Younger Patients
Children require stringent glycemic surveillance to prevent developmental impairments associated with hypo- or hyperglycemia. Parental involvement remains crucial.
Importance of Education for Caregivers and Children
Structured diabetes education benefits both caregivers and young patients by helping them develop self management skills and adhere to treatment plans from an age.
Overdose and Emergency Management with Insulatard FlexPen
Signs and Symptoms of Insulin Overdose
- Severe hypoglycemia
- Neurological disturbances: confusion, seizures, unconsciousness
- Autonomic symptoms: sweating, palpitations, tremors
Immediate First Aid Measures
Prompt administration of fast-acting carbohydrates (e.g., glucose gel, fruit juice) is crucial. Severe cases may require intramuscular glucagon or intravenous dextrose administration.
Medical Interventions for Severe Hypoglycemia
Emergency medical services should be summoned if the patient is unresponsive. Hospitalization may be necessary for glucose infusion and monitoring until stabilization.
Handling Precautions and Safe Disposal of Insulatard FlexPen
Pen Handling Tips to Maintain Sterility
Always store the FlexPen with the cap securely attached. Use sterile needles for each injection and avoid touching the needle hub to maintain aseptic technique.
Needle Usage and Disposal Best Practices
Single-use needles should be discarded immediately after injection in approved sharps containers to minimize infection risks and prevent accidental needle sticks.
Environmental Considerations for Discarding Used Pens
Expired or used FlexPens should be disposed of in accordance with local regulations for biomedical waste, minimizing environmental contamination risks.
Insulatard Flexpen Pen-filled Injection FAQ
- What is insulatard FlexPen used for?
- Which pen is used for insulatard?
- How do you use an insulatard pen?
- What type of insulin is insulatard?
- What are the side effects of insulatard insulin?
- Can insulatard be given at bedtime?
- Where do you inject insulatard?
- How long does an insulatard injection take?
- Should insulatard be given before or after meals?
- Can Insulatard be given IV?
- How long does it take for insulatard to work?
- Can you eat after taking insulin at night?
- What are the common side effects of insulatard?
- What is insulatard Flexpen used for?
- What type of insulin is FlexPen?
What is insulatard FlexPen used for?
Insulatard 100IU/ml Flexpen is prescribed for enhancing blood sugar management in both adults and children diagnosed with type 1 and type 2 diabetes mellitus.
Which pen is used for insulatard?
InnoLet or FlexPen
How do you use an insulatard pen?
Insulatard is usually given through an injection under the skin.
What type of insulin is insulatard?
Isophane (NPH) human insulin suspension
What are the side effects of insulatard insulin?
Feeling sweaty and experiencing tremors or shaking can be signs of nervousness or anxiety; having trouble concentrating and a fast heartbeat
Can insulatard be given at bedtime?
Yes
Where do you inject insulatard?
Thigh
How long does an insulatard injection take?
1½ hours
Should insulatard be given before or after meals?
Before breakfast, evening meal, or bedtime
Can Insulatard be given IV?
No
How long does it take for insulatard to work?
1½ hours
Can you eat after taking insulin at night?
Yes
What are the common side effects of insulatard?
When using insulin therapy, like any medication injection therapy approach may lead to injection site reactions which may comprise pain discomfort redness spots hives itching inflammation bruising or swellings
What is insulatard Flexpen used for?
To enhance the management of blood sugar levels in adults and kids diagnosed with type 1 and type 2 diabetes.
What type of insulin is FlexPen?
Rapid-acting insulin analog