Introduction to Obelit (Orlistat)
Obelit, containing Orlistat, is a potent gastrointestinal lipase inhibitor designed to attenuate the digestion and absorption of dietary fat. It functions directly in the lumen of the gastrointestinal tract, rather than being systematically absorbed. This pharmacological approach enables an energy deficit without central nervous system stimulation.
Fat absorption blockers such as Obelit can alter the metabolic trajectory of weight management. By blocking enzymatic cleavage of triglycerides, less absorbable lipid substrate enters circulation, promoting gradual weight reduction. In many populations, this mechanism is preferable to stimulant-based anorectic therapies.
Orlistat is regulated differently based on jurisdiction. In some markets the 60 mg formulation is available OTC. The 120 mg version (as in Obelit) is generally dispensed under prescription supervision in clinical weight management programs.
Pharmacological Composition and Formulations
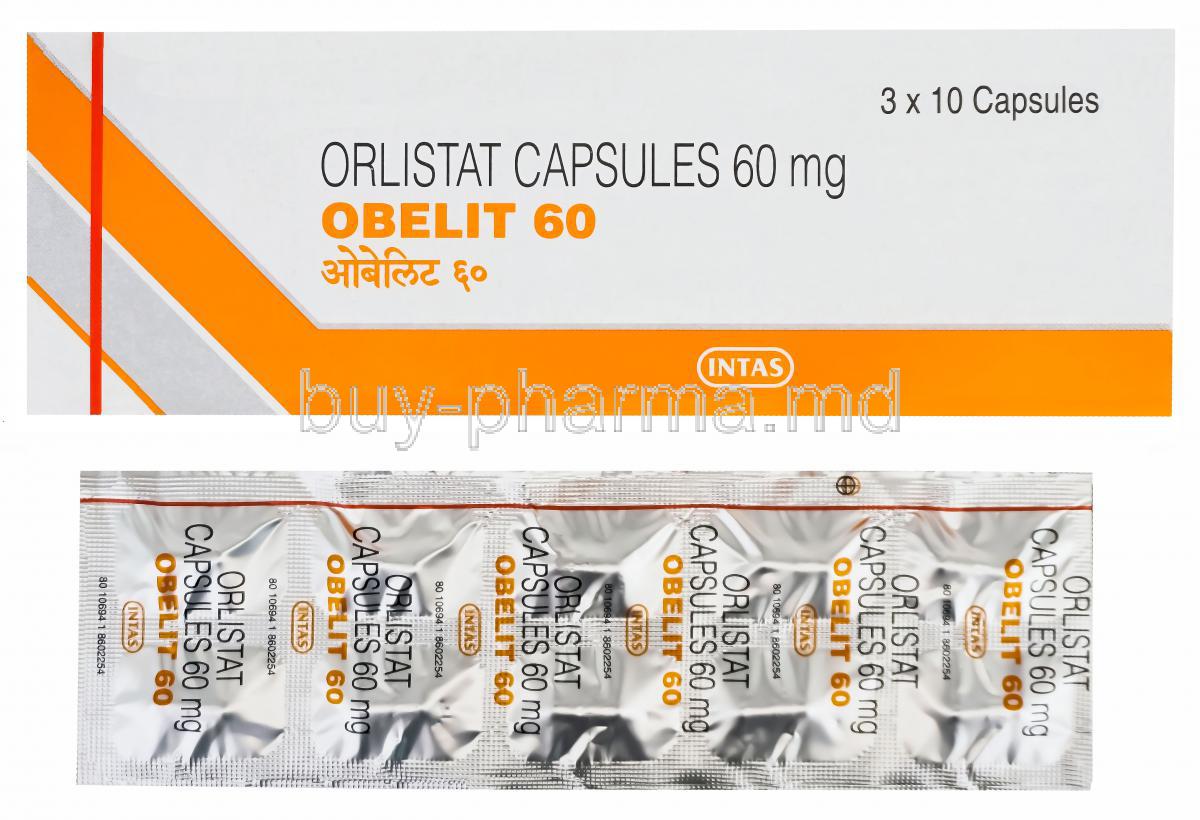
Each Obelit capsule contains Orlistat 120 mg. This concentration optimally inhibits gastrointestinal lipases and yields clinically meaningful fecal fat excretion.
- Direct antagonism of gastric and pancreatic lipases
- High lipid-substrate affinity for maximal therapeutic binding
- Exogenous excipients stabilise capsule integrity
The excipient matrix, while inert, influences stability and disintegration. Obelit capsules are commonly presented as hard-gelatin forms to ensure reliable dissolution kinetics in the duodenum.
Therapeutic Uses and Indications
Orlistat (Obelit) is FDA-approved for:
- Obesity management in adults with BMI ≥30 kg/m²
- Overweight patients with BMI ≥27 kg/m² who present cardiovascular or metabolic risk factors such as:
- Hypertension
- Dyslipidemia
- Type 2 Diabetes Mellitus
It is frequently employed for weight maintenance following successful initial weight loss, as weight regain is physiologically common without pharmacologic support.
Expanded Off-Label Uses
Investigators and clinicians have utilised Orlistat as adjunctive therapy in metabolic cases beyond classic obesity:
- Prediabetes with elevated cardiometabolic risk
- NAFLD / NASH protocols to reduce hepatic lipid load
- Insulin resistance adjunct to lifestyle or metformin therapy
- Weight reduction in PCOS populations
- Metabolic infertility pathways influenced by central adiposity
How Obelit (Orlistat) Works — Mechanism of Action
Orlistat irreversibly inhibits serine residues within gastric and pancreatic lipases. When lipase enzymes lose catalytic binding capacity, triglycerides cannot be hydrolysed into monoacylglycerols and free fatty acids suitable for intestinal absorption.
This causes unabsorbed triglycerides to be excreted via fecal elimination. The cumulative effect is a measurable caloric deficit, allowing gradual fat mass reduction even if caloric intake is not drastically restricted.
Dosage, Timing and Administration Guidelines
Most adults take 120 mg three times daily with main meals that contain fat. If a meal is skipped or fat content is negligible, the corresponding dose should also be skipped.
- Do not exceed recommended daily dose
- Evaluate therapeutic response after several months
- If a dose is missed, do not double the next dose
Side Effects Overview
Because the mechanism is gastrointestinal, most adverse events are localized to the lower GI tract. Many events diminish with a lower lipid diet. Individuals with disciplined dietary fat reduction often report high tolerability.
Common Side Effects (Detailed)
- Steatorrhea or oily stools
- Oily spotting or rectal discharge
- Flatulence with fecal material
- Cramp-like abdominal discomfort
- Progressive depletion of fat-soluble vitamin reserves (A, D, E, K)
Serious Adverse Reactions and Red-Flag Symptoms
- Hepatotoxicity — jaundice, dark urine, severe fatigue
- Pancreatitis — severe upper abdominal pain radiating to the back
- Oxalate nephropathy / kidney stones — flank pain, microhematuria
Drug–Drug Interactions
- Cyclosporine — absorption interference; dose separation strongly advised
- Warfarin — INR instability requires close monitoring
- Levothyroxine — pharmacokinetic separation mandatory for euthyroid stability
- Anticonvulsants — erratic plasma levels possible; clinical vigilance required
Warnings and Important Precautions
Fat-soluble vitamin supplementation becomes necessary in prolonged Orlistat therapy due to reduced micellar lipid absorption. Vitamins A, D, E, and K should be taken at least two hours away from Orlistat to prevent competitive interference. In addition, individuals consuming higher-fat dietary patterns may note greater gastrointestinal turbulence, while low-lipid diets often yield superior tolerability. Patients with chronic undernutrition or borderline caloric intake may face worsening micronutrient insufficiency and should be monitored for malabsorption sequelae.
- Supplement fat-soluble vitamins at medically appropriate intervals
- Modulate dietary fat to fine-tune tolerability
- Evaluate nutrient status periodically in malnourished risk groups
Contraindications
Orlistat is contraindicated in chronic malabsorption syndromes where nutrient impairment already exists as a baseline. The mechanism of lipase inhibition may exacerbate metabolic and nutritional fragility. Individuals with cholestasis should avoid therapy due to altered bile flow and impaired lipophilic nutrient transport. Patients with known hypersensitivity to Orlistat or any capsule component must not initiate treatment.
Careful Administration and Risk-Segment Guidance
Population-specific pharmacovigilance is essential. In diabetes, improved insulin sensitivity or weight reduction may precipitate hypoglycemia, necessitating glucose monitoring and potential dose adjustments of antidiabetic agents. Thyroid disease patients may encounter altered levothyroxine absorption requiring spaced dosing and biochemical checks. Individuals with a history of nephrolithiasis need surveillance for oxalate deposition due to increased intestinal oxalate uptake.
- Glucose trend monitoring in diabetes mellitus
- TSH / free T4 checks in thyroid disease
- Nephrolithiasis risk reassessment in predisposed populations
Administration to Elderly Patients
The elderly require a nuanced approach, as gastrointestinal tolerance may vary and polypharmacy is common. Frailty indexing and baseline nutritional scoring should be considered before initiating Orlistat. Very advanced age groups may experience disproportionate GI burdens, especially when lipid intake fluctuates.
Administration to Pregnant Women and Nursing Mothers
Orlistat is not recommended during pregnancy. Weight loss is not advised in gestation since fetal micronutrient demands and placental development require metabolic stability. Lactation data is insufficient, and the effect of Orlistat-linked lipid malabsorption on breastmilk composition remains unknown. Pharmacological weight loss should therefore be deferred until maternal nutritional equilibrium is restored postpartum.
- Prenatal use discouraged regardless of BMI
- Lactation exposure uncertain
- Use should resume only after breastfeeding cessation if clinically valid
Administration to Children and Adolescents
Younger populations may only use Orlistat under specialist oversight. Growth plates, endocrine maturation, and neurodevelopment are highly sensitive to micronutrient balance, thus requiring rigorous dietary supervision. Vitamin status should be periodically verified, as adolescent compliance variability can compound deficiency risk.
Overdosage and Emergency Management
Overdose typically produces an amplification of gastrointestinal adverse effects rather than systemic toxicity. Supportive care is the standard approach, and no specific antidote exists. Emergency evaluation becomes imperative if severe abdominal pain, signs of dehydration, or uncontrollable steatorrhea occur.
- Monitor hydration and electrolytes
- No role for forced emesis or charcoal absorption
- Consult emergency care for escalating symptoms
Handling and Storage Precautions
Store capsules at controlled room temperature, protected from humidity and excessive heat. Avoid direct ultraviolet exposure to maintain capsule integrity. Containers should be sealed tightly and kept inaccessible to children to mitigate accidental ingestion. High-moisture settings like bathrooms are not ideal for long-term storage.
- Maintain temperature stability
- Shield from sunlight
- Store away from minors