Pneumo 23 Vanccine
- 1. Introduction to Pneumo 23 Vaccine
- 2. Medical Uses of Pneumo 23 Vaccine
- 3. Off-label Uses of Pneumo 23 Vaccine
- 4. How Pneumo 23 Vaccine Works
- 5. Composition and Formulation of Pneumo 23 Vaccine
- 6. Dosage and Administration Guidelines
- 7. Common and Serious Side Effects of Pneumo 23 Vaccine
- 8. Drug and Vaccine Interactions
- 9. Contraindications and Populations Not Recommended
- 10. Warnings and Special Precautions
- 11. Careful Administration in Special Populations
- 11.1 Administration to Elderly Patients
- Immunosenescence and Response to Pneumo 23
- Adjusted Risk-Benefit Assessment in Frail Older Adults
- 11.2 Administration During Pregnancy and Lactation
- Safety Profile in Pregnant Women
- Transfer of Antibodies Through Breastmilk
- WHO and CDC Recommendations
- 11.3 Use in Pediatric Populations
- Approved Age Group for Administration
- Not Recommended for Children Below 2 Years
- Comparison with Conjugate Vaccines for Infants
- 12. Overdose and Emergency Measures
- 13. Storage and Handling of Pneumo 23 Vaccine
- 14. Handling Precautions for Healthcare Professionals
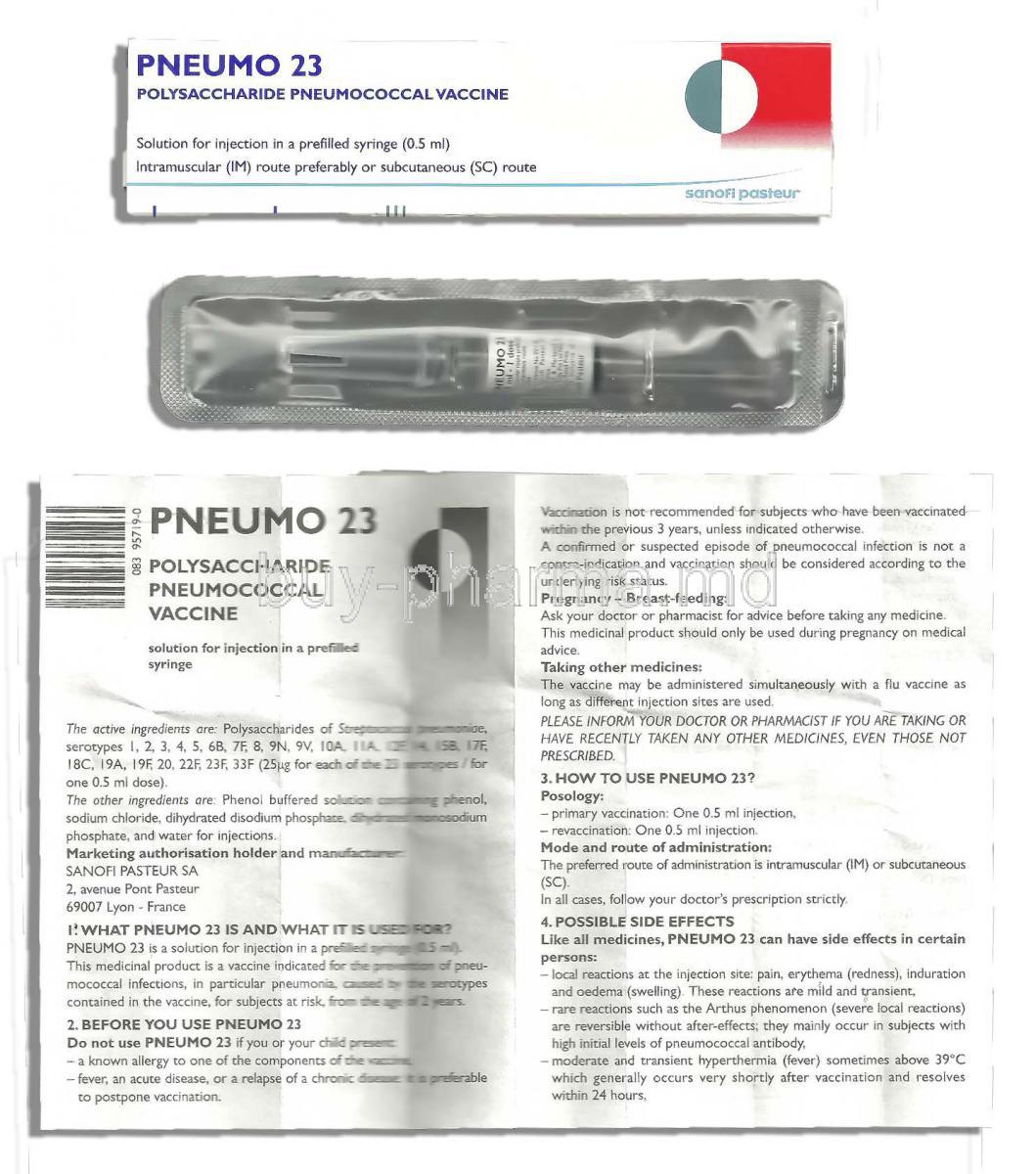
1. Introduction to Pneumo 23 Vaccine
What is Pneumo 23 Vaccine?
The Pneumovax 23 Vaccine, also known as PPSV23, is a shot that helps protect against 23 strains of Streptococcus bacteria that can cause bacterial illnesses, such as pneumonia and meningitis, in adults and those at higher risk of infection.
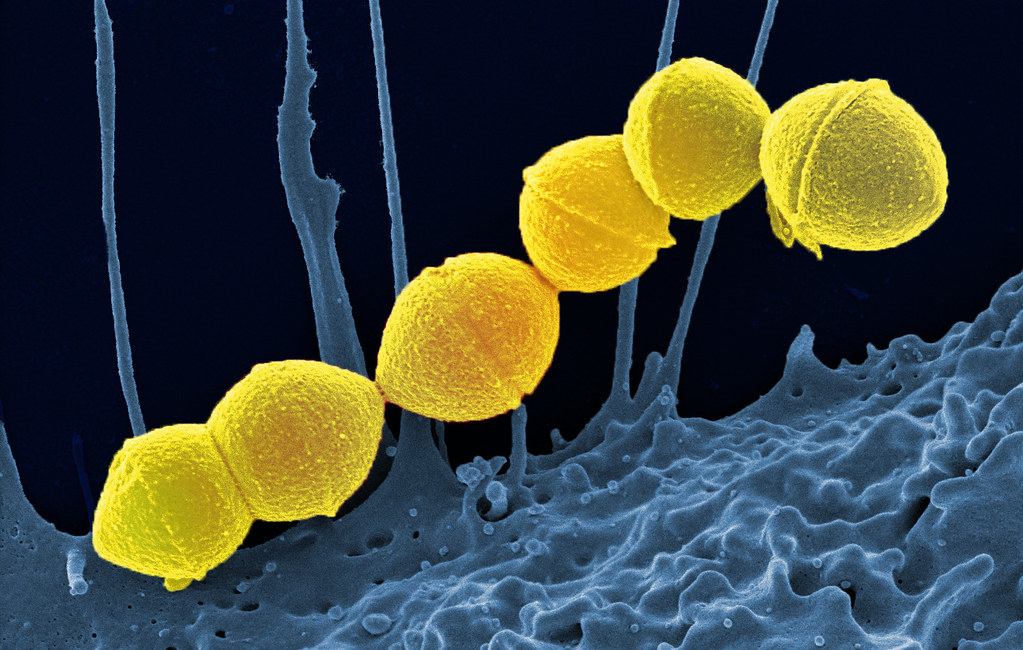
Overview of Pneumococcal Diseases
Pneumococcal diseases are invasive bacterial infections that can lead to life-threatening conditions:
- Lobar pneumonia and bronchopneumonia
- Bacteremia and septic shock
- Meningitis with neurological complications
- Otitis media and sinusitis in less severe presentations
Importance of Pneumococcal Vaccination
Pneumococcal infections remain a significant public health concern, especially among older adults, immunocompromised individuals, and those with chronic illnesses. Vaccination with Pneumo 23 helps:
- Reduce morbidity and mortality associated with invasive pneumococcal disease (IPD)
- Lower hospitalization rates and antibiotic resistance
- Prevent secondary complications from respiratory viruses such as influenza
Differences Between Pneumo 23 and Other Pneumococcal Vaccines (e.g., PCV13)
Pneumo 23 (PPSV23) covers a broader range of pneumococcal serotypes compared to PCV13, which is a conjugated vaccine:
- PPSV23: 23 serotypes; T-cell independent; more effective in adults
- PCV13: 13 serotypes; T-cell dependent; more immunogenic in children under 2
- Pneumo 20 vs 23: Pneumo 20 protects against 20 serotypes, while Pneumo 23 protects against 23
2. Medical Uses of Pneumo 23 Vaccine
Prevention of Pneumococcal Infections in Adults
Use in High-Risk Groups
Recommended for:
- Individuals with diabetes, asthma, or heart disease
- Smokers or people with chronic lung conditions

Use in Immunocompromised Patients
Essential in patients with:
- HIV/AIDS
- Hematologic malignancies
- Organ transplantation or chemotherapy exposure
Use in Travelers to High-Risk Areas
When traveling to regions where outbreaks of disease are common or medical services are limited, it's typically recommended for visitors to get the pneumonia 23 vaccine as a preventive measure.
Prophylaxis in Post-Splenectomy Patients
3. Off-label Uses of Pneumo 23 Vaccine
Prevention of Secondary Bacterial Infections in Viral Respiratory Illnesses
Use in Certain Autoimmune Disorders
Administered in autoimmune conditions such as:
- Systemic lupus erythematosus (SLE)
- Rheumatoid arthritis especially before initiating immunosuppressive therapy
Experimental Use in Elderly Patients with Recurrent Bronchitis or COPD
4. How Pneumo 23 Vaccine Works
Mechanism of Action
Polysaccharide Composition and Its Role in Immunogenicity
The purified polysaccharide antigens elicit a B-cell mediated response. Unlike conjugated vaccines, PPSV23 does not rely on T-cell activation, making it less effective in infants.
Onset of Action and Duration of Immunity
Protective antibody levels are generally achieved within 2 to 3 weeks post-vaccination. Immunity may persist for 5 to 10 years depending on the individual's health status.
Role of Memory B-Cells and T-Cell Independent Response
Induces short-lived memory B-cell responses. Lack of T-cell involvement limits its ability to generate long-lasting immunity or robust response upon repeated exposure.

5. Composition and Formulation of Pneumo 23 Vaccine
Active Ingredients and Pneumococcal Serotypes Covered
Contains purified capsular polysaccharides from 23 serotypes, including 1, 3, 4, 6B, 9V, 14, 19F, and 23F covering approximately 85-90% of serotypes causing IPD globally.
Excipients and Preservatives
Includes phenol as a preservative and isotonic sodium chloride solution for stabilization.
Available Formulations and Packaging
Supplied in single-dose pre-filled syringes and vials. Requires no reconstitution before administration.
6. Dosage and Administration Guidelines
Recommended Dosage for Adults and Children Over 2 Years
Single 0.5 mL dose administered intramuscularly or subcutaneously. Not recommended for children under 2 due to limited immune response.
Route of Administration
Can be administered either intramuscularly (preferably deltoid) or subcutaneously in patients with bleeding disorders.
Pneumo 23 vaccine schedule
Revaccination is considered:
- After 5 years in high-risk individuals
- 10 years post-primary dose in elderly or immunocompromised patients
Co-administration with Other Vaccines
Can be co-administered with inactivated influenza or COVID-19 vaccines at different anatomical sites using separate syringes.
7. Common and Serious Side Effects of Pneumo 23 Vaccine
7.1 Common Side Effects
Typical adverse effects are mild and transient:
- Redness, pain, or swelling at the injection site
- Fever, fatigue, or general malaise
- Myalgia or headache

7.2 Serious and Rare Side Effects
Although rare, severe reactions have been documented:
- Anaphylaxis requiring emergency intervention
- Arthus-type local hypersensitivity with prior dosing
- Neurologic events such as Guillain-Barre Syndrome (extremely rare)
8. Drug and Vaccine Interactions
Interactions with Immunosuppressive Therapies
Individuals taking corticosteroids or undergoing chemotherapy or treatments may experience a decrease in response to vaccines. The optimal timing for vaccination is before starting these therapies.
Timing Considerations with Other Vaccines
If co-administered with PCV13, PCV13 should be given first, followed by PPSV23 at least 8 weeks later to optimize immune response.
Effects of Concomitant Use of Corticosteroids
High-dose systemic corticosteroids may blunt vaccine response. Temporarily withholding immunosuppressants may be considered in some cases.
Impact of Chemotherapy and Radiation Therapy
Vaccination should be planned during remission or before the initiation of cytotoxic therapy for optimal efficacy.
9. Contraindications and Populations Not Recommended
Known Hypersensitivity to Any Component of the Vaccine
Contraindicated in individuals with a documented allergy to phenol or any of the vaccine components.
Previous Severe Allergic Reaction to Pneumo 23
Acute Febrile Illness at the Time of Vaccination
It's best to wait on getting vaccinated if you're dealing with an illness until your symptoms clear up so that any reactions after the vaccine aren't mistaken for something else.
10. Warnings and Special Precautions
Risk of Hypersensitivity in Individuals with a History of Allergies
Individuals with a known history of hypersensitivity to vaccine components, such as phenol or polysaccharides, may be at elevated risk for allergic reactions following Pneumo 23 administration.
- Pre-vaccination screening is advised to identify at-risk patients.
- Patients with atopic conditions or previous anaphylaxis should be evaluated cautiously.

Caution in Patients with Thrombocytopenia or Bleeding Disorders
For individuals with bleeding tendencies, especially those with thrombocytopenia or hemophilia, care must be taken to minimize intramuscular bleeding. In such cases:
- Subcutaneous administration is often preferred over intramuscular injection.
- Applying firm pressure at the injection site for several minutes can reduce hematoma formation.
Monitoring for Anaphylaxis Post-Injection
Although rare, anaphylactic reactions may occur following vaccination. It is essential that:
- Patients remain under observation for at least 15-30 minutes post-injection.
- Emergency resuscitation equipment and epinephrine are immediately available onsite.
Importance of Medical Supervision During Administration
The vaccine should be administered by trained healthcare professionals in a clinical setting. This ensures:
- Proper injection technique is used.
- Immediate medical intervention is available in the event of adverse events.
11. Careful Administration in Special Populations
11.1 Administration to Elderly Patients
Immunosenescence and Response to Pneumo 23
In elderly patients, immune responsiveness is diminished due to immunosenescence. This may result in:
- Reduced antibody titers following vaccination.
- Lower duration of protection compared to younger adults.
Adjusted Risk-Benefit Assessment in Frail Older Adults
For frail elderly patients or those with multiple comorbidities, vaccination should be individualized:
- Assess functional status and life expectancy.
- Evaluate potential benefits versus risks of adverse effects.
11.2 Administration During Pregnancy and Lactation
Safety Profile in Pregnant Women
While safety data are limited, current evidence suggests that Pneumo 23 is not associated with teratogenic risks when administered during pregnancy, particularly in the second or third trimester.

Transfer of Antibodies Through Breastmilk
Post-vaccination maternal antibodies may pass into breast milk, offering potential passive protection to infants. No adverse effects have been documented in breastfed neonates.

WHO and CDC Recommendations
Both WHO and CDC advise pneumococcal vaccination for pregnant individuals with high-risk conditions such as chronic heart, lung, or liver disease, or diabetes.
11.3 Use in Pediatric Populations
Approved Age Group for Administration
Pneumo 23 is approved for children aged 2 years and older, particularly those with chronic illnesses or immunocompromising conditions.
Not Recommended for Children Below 2 Years
The vaccine's polysaccharide formulation does not elicit an adequate immune response in children under 2 years due to immature B-cell function.
Comparison with Conjugate Vaccines for Infants
For infants and toddlers, conjugate vaccines such as PCV13 are preferred:
- Stronger and longer-lasting immunity through T-cell dependent mechanisms.
- Greater protection against nasopharyngeal carriage and community transmission.
12. Overdose and Emergency Measures
Possibility and Symptoms of Vaccine Overdose
Although vaccine overdose is rare due to controlled administration, unintentional repeated doses may occur. Symptoms may include:
- Exaggerated local reactions (e.g., swelling, redness, tenderness)
- Fever, malaise, or immune-mediated inflammation
Recommended Management of Accidental Double Dosing
If an overdose is suspected:
- Monitor the patient for 24-48 hours.
- Manage symptoms with supportive care and analgesics if necessary.
Reporting to Pharmacovigilance Authorities
Any suspected overdose or adverse reaction should be promptly reported to local health authorities or national vaccine safety monitoring systems for documentation and evaluation.
13. Storage and Handling of Pneumo 23 Vaccine
Recommended Temperature Range and Conditions
Pneumo 23 must be stored under refrigerated conditions:
- Maintain a temperature between 2°C and 8°C (36°F and 46°F).
- Do not freeze, as freezing damages the polysaccharide components.
Shelf Life and Expiry Considerations
Each batch has a designated expiry date that must be adhered to:
- Expired vials must not be administered.
- Opened vials should be used promptly or discarded as per institutional protocols.
Avoidance of Freezing and Light Exposure
Freezing renders the vaccine inactive. Additionally, protect the vials from direct sunlight and excessive heat, which may compromise vaccine efficacy.
14. Handling Precautions for Healthcare Professionals
Use of Sterile Equipment
Strict aseptic conditions are essential. Use only sterile, single-use needles and syringes for each patient to avoid contamination and cross-infection.
Aseptic Technique During Administration
Adherence to aseptic injection technique ensures safe administration and minimizes injection site infections and post-vaccination complications.
Proper Disposal of Vaccine Vials and Syringes
All used or expired vials, syringes, and needles must be disposed of in designated sharps containers in compliance with biomedical waste regulations.
Documentation and Record Keeping
Thorough documentation of:
- Batch number and expiry date
- Date and site of administration
- Any adverse reactions or events
is essential for pharmacovigilance and future reference.
Pneumo 23 Vanccine FAQ
- What is the pneumo 23 Vaccine used for?
- How often should PNEUMOVAX 23 be given?
- What is the difference between pneumonia vaccine 13 and 23?
- Is Prevnar 20 better than Pneumo 23?
- How long does Pneumo 23 last?
- Is Pneumovax 23 good for life?
- Does Pneumovax need a booster?
- Is Pneumovax a lifetime vaccine?
- Who should get pneumococcal 23?
- Which is better PCV13 or PPSV23?
- What are the side effects of pneumonia vaccine 23?
- How many pneumonia 23 shots do you need?
- Is Pneumovax 23 a live vaccine?
- Who is eligible for Pneumovax 23?
- How many years between Prevnar 13 and Pneumovax 23?
- When should I repeat Pneumovax 23?
- Is Pneumovax 23 safe?
- How long has Pneumovax 23 been available?
- Is Pneumo 23 recommended?
- What are the benefits of Pneumovax 23?
- What happens if you get Pneumovax 23 twice?
- Do I need both PCV13 and PPSV23?
What is the pneumo 23 Vaccine used for?
The Pneumovax 23 vaccine guards against pneumococcus infections that lead to illnesses like ear infections and pneumonia, among others, by prompting your body to generate antibodies for defense against these harmful bacteria.
How often should PNEUMOVAX 23 be given?
Adults are typically advised to wait a year between intervals for vaccinations; in cases where individuals have an immunocompromising condition or specific medical issues, like cochlear implants or cerebrospinal fluid leaks, an 8-week interval could be considered.
What is the difference between pneumonia vaccine 13 and 23?
PCV13 protects against 13 strains of pneumococci, while PPSV23 shields against 23 strains in total.
Is Prevnar 20 better than Pneumo 23?
Prevnar 20 is believed to offer greater protection against the targeted strains compared to Pneumovax 23. Its benefits are expected to endure for a longer duration—while Pneumovax 23s efficacy typically diminishes after approximately 5 to 6 years.
How long does Pneumo 23 last?
Taking one to three shots of the pneumonia vaccine should provide protection for a lifetime.
Is Pneumovax 23 good for life?
Yes
Does Pneumovax need a booster?
Some adults and certain high-risk children who previously received vaccines might require a booster shot.
Is Pneumovax a lifetime vaccine?
For individuals aged 50 and above, in the majority of cases, a solitary vaccination dose will grant immunity.
Who should get pneumococcal 23?
Kids under five and individuals aged 50 and above.
Which is better PCV13 or PPSV23?
PC13 appears to have the ability to create lasting immunity.
What are the side effects of pneumonia vaccine 23?
According to the data, the common side effects observed were swelling at the injection site, headaches, redness at the injection site, fatigue or weakness , and muscle pain.
How many pneumonia 23 shots do you need?
Two
Is Pneumovax 23 a live vaccine?
No
Who is eligible for Pneumovax 23?
infants and children aged under 5 years and older
How many years between Prevnar 13 and Pneumovax 23?
6–12 months
When should I repeat Pneumovax 23?
If you face a chance of a pneumococcal infection
Is Pneumovax 23 safe?
Yes
How long has Pneumovax 23 been available?
1983
Is Pneumo 23 recommended?
Yes
What are the benefits of Pneumovax 23?
Prevents pneumococcus bacterial infections
What happens if you get Pneumovax 23 twice?
It is not harmful.
Do I need both PCV13 and PPSV23?
No