Introduction to Tacrotor Ointment
Overview of Tacrotor Ointment
Tacrotor Ointment is a dermatological preparation designed for the management of chronic inflammatory skin conditions. It provides targeted relief from persistent irritation, redness, and itching without the risks typically associated with long-term steroid use. This medication has become an essential option for patients seeking sustained therapy with a favorable safety profile.
Classification as a Topical Calcineurin Inhibitor
The ointment belongs to the class of topical calcineurin inhibitors, which act by modulating immune responses in the skin. Unlike corticosteroids, it does not induce skin thinning, making it particularly suitable for delicate regions such as the face and intertriginous areas.
Approved Indications in Dermatology
Clinicians prescribe Tacrotor primarily for:
- Atopic dermatitis in both adults and children
- Moderate to severe eczema unresponsive to first-line therapies
- Chronic flare-up prevention in sensitive zones
Benefits over Traditional Corticosteroid Therapy
The advantages are significant:
- Minimal risk of skin atrophy or striae
- Safe for long-term use under medical guidance
- Effective in regions where corticosteroids may be contraindicated
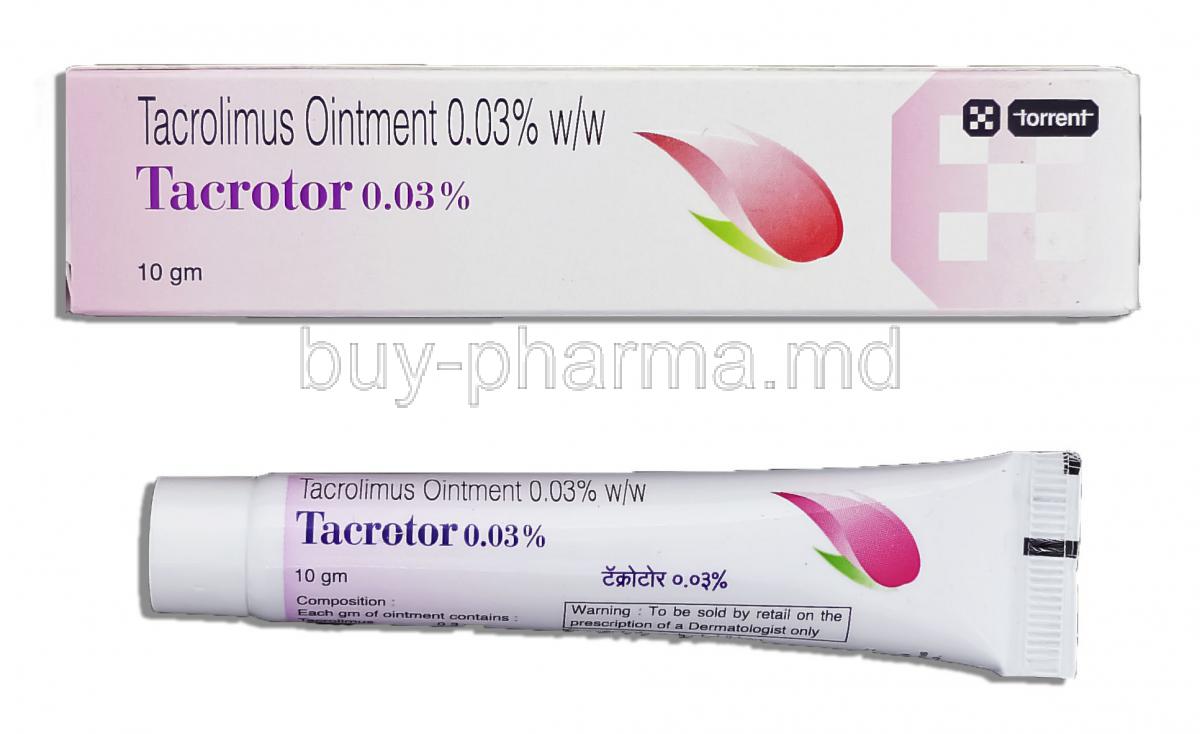
Composition and Formulation
Active Ingredient: Tacrolimus
The pharmacologically active compound is Tacrolimus, a macrolide lactone with potent immunomodulatory properties.
Available Strengths and Formulations
Tacrotor is offered in two main concentrations to match disease severity:
- 0.03% ointment – typically prescribed for children
- 0.1% ointment – reserved for adults and severe cases
Inactive Ingredients and Excipients
Alongside the active drug, the formulation includes stabilizers and emollient bases that ensure optimal skin penetration and consistency.
Packaging Information
The ointment is usually supplied in sealed aluminum tubes, designed to protect the integrity of the preparation from light and moisture.
Mechanism of Action: How Tacrotor Works
Role as a Calcineurin Inhibitor
Tacrolimus binds to intracellular proteins, creating a complex that inhibits the phosphatase activity of calcineurin. This process curtails downstream inflammatory signaling.
Suppression of T-cell Activation and Cytokine Release
By dampening T-cell activity, the ointment reduces the secretion of pro-inflammatory cytokines such as interleukin-2, thus attenuating the cascade of immune-driven skin damage.
Reduction of Skin Inflammation and Immune Response
The outcome is a noticeable decline in erythema, pruritus, and lichenification. Patients often report improved comfort and quality of life.
Comparison with Corticosteroid Mechanisms
While corticosteroids blunt inflammation through broad hormonal effects, tacrolimus achieves its impact by specifically modulating immune cell activation, minimizing systemic repercussions.
Therapeutic Uses
Atopic Dermatitis (Eczema) Management
The ointment is a cornerstone treatment for eczema, particularly in individuals who cannot tolerate steroids. It provides symptomatic relief and helps restore skin barrier function.
Treatment of Chronic Lichenified Eczema
Thickened, persistent lesions often respond well due to tacrolimus’s ability to reduce immune hyperactivity without aggravating skin texture.
Use in Corticosteroid-Sensitive Areas
Tacrotor is uniquely suited for delicate sites including:
- Facial skin around the eyes and mouth
- Neck and upper chest
- Skin folds prone to irritation
Maintenance Therapy to Prevent Flare-Ups
Intermittent application has demonstrated efficacy in prolonging remission and minimizing relapses.
Off-Label and Emerging Uses
Vitiligo Repigmentation Therapy
Tacrolimus has shown promise in stimulating melanocyte activity, assisting in repigmentation of depigmented patches.
Seborrheic Dermatitis Treatment
Patients with resistant seborrheic dermatitis may benefit from localized application, particularly in facial areas.
Lichen Planus (Oral and Cutaneous)
Clinical data support its efficacy in reducing discomfort and lesion severity in both oral mucosa and skin manifestations.
Psoriasis Management in Sensitive Areas
Unlike potent steroids, Tacrotor can be applied to intertriginous or facial psoriatic plaques with reduced risk of adverse outcomes.
Post-Transplant Cutaneous Graft-Versus-Host Disease
Topical tacrolimus has emerged as an adjunct in cases where standard immunosuppression proves insufficient.
Other Inflammatory Dermatoses Under Clinical Investigation
Research continues into applications for alopecia areata, rosacea, and chronic urticaria.
Dosage and Administration
Recommended Starting Dose for Adults and Children
Adults often commence with the 0.1% ointment, while children are typically prescribed the 0.03% concentration.
Application Frequency and Duration of Therapy
Usual practice involves twice-daily application until lesions subside, followed by maintenance as directed by a healthcare provider.
Tapering and Maintenance Strategies
Once control is achieved, frequency may be reduced to intermittent use to sustain remission.
Guidelines for Long-Term Use
Long-term therapy is permissible, provided regular dermatological reviews are conducted to monitor safety.
Instructions for Use in Sensitive Skin Areas
Apply a thin, uniform layer to affected zones, avoiding excessive rubbing. Ointment should not be occluded unless specifically instructed.
Special Population Considerations
Administration to Children
- Approved age groups: safe for use in children over two years of age.
- Safety and efficacy: clinical studies confirm effectiveness with careful monitoring.
- Long-term precautions: prolonged use requires vigilance due to limited pediatric data.
Administration to Elderly Patients
- Absorption rates may differ in older skin
- Adverse reactions are not substantially increased, but monitoring remains essential
- Dose adjustments are seldom necessary but should be individualized
Administration to Pregnant Women
- Fetal exposure poses theoretical risks
- Animal data show teratogenicity at high doses
- Use is reserved for cases where benefits outweigh potential harm
Administration to Nursing Mothers
- Tacrolimus can be excreted in breast milk
- Breastfeeding mothers should avoid application to the breast area
- Clinical consultation is vital before continuation during lactation
Side Effects of Tacrotor Ointment
Common Side Effects
Tacrotor Ointment, while effective, may cause localized discomfort in certain patients. These effects are generally mild and transient but should be acknowledged.
- Burning, stinging, or itching at the site of application, particularly during the initial days of therapy
- Temporary skin redness and irritation that subsides with continued use
- Occasional systemic symptoms such as headache, fatigue, or mild flu-like sensations
Serious Adverse Reactions
Although infrequent, severe reactions demand immediate medical evaluation. These risks highlight the necessity of careful monitoring during prolonged therapy.
- Increased susceptibility to viral skin infections such as herpes simplex or varicella
- Rare systemic absorption, leading to immune modulation beyond the intended site
- Potential long-term association with skin malignancies and lymphoproliferative disorders
Warnings and Important Precautions
FDA Black Box Warning Regarding Malignancy Risk
Tacrotor carries a black box warning highlighting the theoretical risk of lymphoma and skin cancers. This necessitates cautious, supervised use.
Avoidance of Prolonged Continuous Use
Extended, unmonitored application should be avoided. Intermittent or short-term therapy is preferred to mitigate risks.
Sun Exposure Precautions and UV Protection Advice
Patients are advised to limit sun exposure and employ protective measures:
- Use of broad-spectrum sunscreens
- Wearing protective clothing
- Avoidance of tanning beds or artificial UV sources
Restrictions on Use with Occlusive Dressings
Occlusive bandages or wraps should not be applied over treated areas, as they may increase systemic absorption and adverse effects.
Avoidance of Alcohol Consumption
Some patients experience facial flushing when consuming alcohol during therapy. Limiting alcohol intake can prevent this reaction.
Contraindications
- Known hypersensitivity to tacrolimus or any formulation excipients
- Use in immunocompromised patients unless under strict medical supervision
- Contraindicated in individuals with rare genetic skin barrier defects, such as Netherton’s syndrome
Drug and Substance Interactions
Interaction with Other Topical Immunosuppressants
Concurrent use with similar agents may heighten immunosuppressive effects and infection risk.
Risks with Systemic Tacrolimus or Cyclosporine
Topical and systemic immunosuppressants should not be combined without specialized guidance due to potential cumulative toxicity.
Influence of Strong CYP3A4 Inhibitors
Drugs that inhibit CYP3A4 enzymes can elevate tacrolimus concentrations, even topically, warranting caution.
Caution with Concurrent Phototherapy
Combining Tacrotor with UV-based therapies can enhance carcinogenic risk and should be approached conservatively.
Careful Administration and Monitoring
- Regular dermatological evaluations to assess therapeutic response and detect early adverse effects
- Monitoring for lymphadenopathy or persistent, unusual skin lesions
- Laboratory investigations in long-term users to exclude systemic involvement
- Gradual transition from corticosteroids to tacrolimus, guided by clinical judgment
Overdosage and Toxicity
Risk of Systemic Absorption in Case of Accidental Ingestion
If ingested, tacrolimus may cause systemic immunosuppression, necessitating emergency intervention.
Symptoms of Overdose and Systemic Immunosuppression
Signs include increased infection susceptibility, gastrointestinal discomfort, or neurological disturbances.
Emergency Management Protocols
Supportive treatment, gastric decontamination if appropriate, and monitoring of vital parameters are central to management.
Storage and Handling Precautions
- Store at controlled room temperature, away from direct heat and moisture
- Observe shelf life; typically effective until the expiration date printed on the tube
- Ensure proper closure after use to prevent contamination
- Dispose of unused or expired ointment according to pharmaceutical waste guidelines
Conclusion
Tacrotor Ointment represents a significant advancement in dermatological therapy, offering potent efficacy with a distinct safety profile. The balance between therapeutic benefit and potential risks demands judicious use under professional supervision. With continued research, the role of tacrolimus in immunomodulatory dermatology is expected to expand, providing patients with safer, targeted options for chronic skin conditions.