Alvesco Inhaler
- 1. Introduction to Alvesco Inhaler (Ciclesonide HFA)
- 2. Composition and Pharmaceutical Formulation
- 3. Mechanism of Action: How Alvesco Works
- 4. FDA-Approved Therapeutic Uses
- 5. Emerging and Off-Label Applications
- Asthma Management in Children Aged 2–11 Years
- COPD with Eosinophilic Phenotype and Mixed AsthmaâÂÂCOPD Overlap
- Non-Cystic Fibrosis Bronchiectasis and Chronic Cough Syndromes
- Alvesco Inhaler COVID-19-Related Airway Inflammation and Post-Viral Cough
- Seasonal Allergic Rhinitis via Intranasal Ciclesonide Formulation
- 6. Alvesco Dosage and Administration Guidelines
- 7. Handling Precautions and Proper Inhalation Technique
- 8. Storage and Stability Information
- 9. Drug and Product Interactions
- 10. Alvesco Inhaler Side Effects
- 11. Contraindications
- 12. Warnings and Important Precautions
- 13. Careful Administration in Special Populations
- 14. Overdosage and Emergency Management
- 15. Patient Counseling and Adherence Strategies
1. Introduction to Alvesco Inhaler (Ciclesonide HFA)
Overview and Position in Asthma Treatment Guidelines
Alvesco Inhaler, containing the corticosteroid ciclesonide, is a maintenance inhaler indicated for the long-term control of asthma. Recognized by international asthma management protocols such as GINA (Global Initiative for Asthma), Alvesco is typically introduced as a controller medication in patients whose symptoms are not adequately managed with short-acting bronchodilators alone.
Key Benefits of Lung-Activated Corticosteroid Delivery
Unlike conventional systemic steroids, Alvesco delivers targeted therapy directly to the lungs. The prodrug nature of ciclesonide ensures activation only in the airway tissue, significantly reducing the risk of systemic side effects. This targeted approach enhances patient tolerability and long-term adherence.
2. Composition and Pharmaceutical Formulation
Active Ingredient: Ciclesonide: Chemical Structure and Class
Ciclesonide is a non-halogenated corticosteroid with anti-inflammatory properties. It belongs to the glucocorticoid class and is classified as a prodrug that becomes pharmacologically active after enzymatic cleavage in lung tissue to des-ciclesonide.
Propellant, Surfactants, and Inactive Excipients
Alvesco is formulated with hydrofluoroalkane (HFA-134a) as a propellant, providing environmentally safe aerosolization. It does not contain chlorofluorocarbons (CFCs). The formulation is free from preservatives, ethanol, and lactose, making it suitable for patients with sensitivities.
Hydrofluoroalkane (HFA) Technology vs. CFC Legacy Inhalers
HFA propellants offer improved pulmonary deposition, enhanced drug stability, and eco-friendliness. Compared to older CFC inhalers, HFA-based formulations produce a softer plume and do not contribute to ozone depletion.
Alvesco inhaler vs albuterol
Alvesco (ciclesonide) is a type of corticosteroid that works gradually to reduce inflammation in the lungs and help maintain airways over time for improved breathing comfort and ease of respiration in individuals with respiratory conditions like asthma or COPD (Chronic Obstructive Pulmonary Disease). On the other hand, short-term relief inhalers, such as albuterol (commonly referred to as Ventolin), are designed to provide relief by rapidly opening up the airways during sudden breathing difficulties or asthma attacks.
Alvesco inhaler vs symbicort
Alvesco consists of one corticosteroid called ciclesonide to decrease inflammation in the airways; whereas Symbicort pairs an inhaled corticosteroid (budesonide ) with a long-lasting bronchodilator (formoterol) to manage inflammation and widen airways at the same time. Alvesco is specifically designed for asthma management only; on the other hand, Symbicort is used for treating both asthma and COPD, providing the advantage of a bronchodilator component.
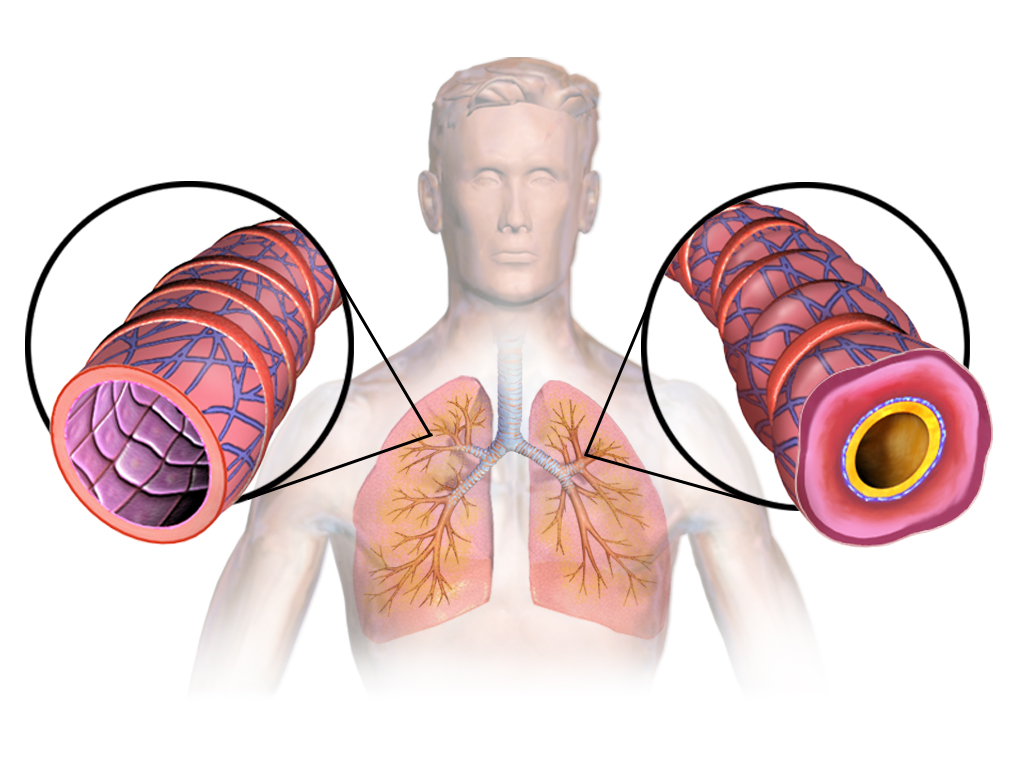
3. Mechanism of Action: How Alvesco Works
Conversion to Active Metabolite (Des-Ciclesonide) in the Airways
Ciclesonide is converted in the epithelial cells of the lungs to its active metabolite, des-ciclesonide. This conversion is catalyzed by esterase enzymes and ensures localized anti-inflammatory activity.
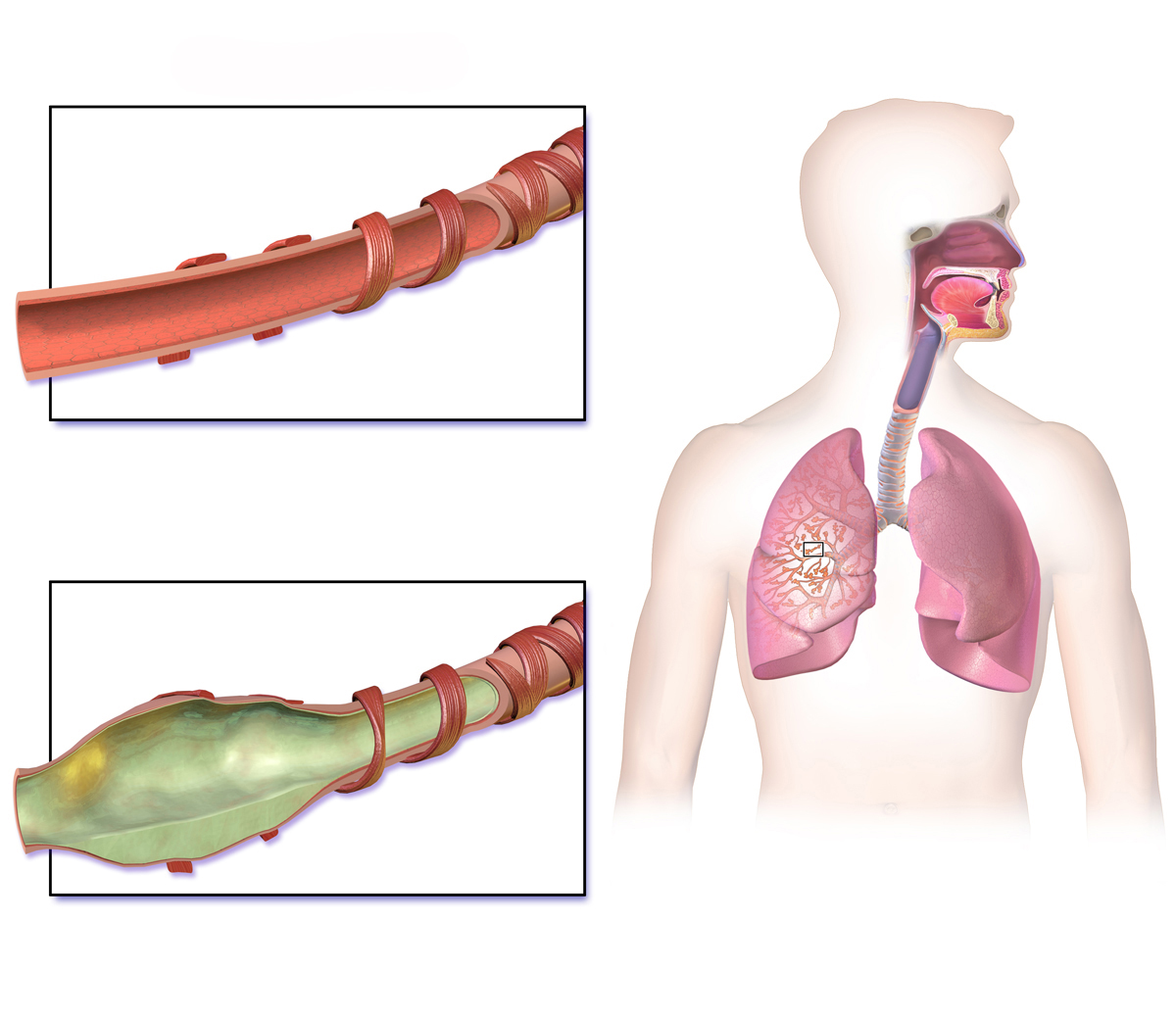
Glucocorticoid Receptor Binding and Anti-Inflammatory Cascade
Des-ciclesonide binds with high affinity to intracellular glucocorticoid receptors. This interaction modulates gene expression, resulting in the inhibition of pro-inflammatory cytokines, suppression of eosinophilic activity, and reduced airway hyperresponsiveness.
Selective Pulmonary Activation and Reduced Systemic Exposure
The design of ciclesonide minimizes systemic corticosteroid exposure. Its low oral bioavailability and strong first-pass hepatic metabolism further reduce the likelihood of systemic adverse effects, including growth suppression and adrenal axis disruption.
4. FDA-Approved Therapeutic Uses
Maintenance Treatment of Persistent Asthma in Adults
Alvesco is approved for the prophylactic management of persistent asthma in adult patients. It is intended for daily use and is not effective for immediate symptom relief.

Step-Down Therapy Following Oral Corticosteroid Taper
Alvesco is also utilized during step-down protocols, where systemic corticosteroids are tapered, and inhaled corticosteroids are introduced to maintain disease control.
Pediatric Asthma Control in Patients in 12 Years
In adolescents aged 12 and above, Alvesco is indicated for the maintenance treatment of asthma, helping reduce the frequency of exacerbations and reliance on rescue medications.
5. Emerging and Off-Label Applications
Asthma Management in Children Aged 2–11 Years

COPD with Eosinophilic Phenotype and Mixed AsthmaâCOPD Overlap
In patients with overlapping features of asthma and chronic obstructive pulmonary disease (COPD), particularly those with eosinophilic inflammation, Alvesco may serve as part of a combination regimen.
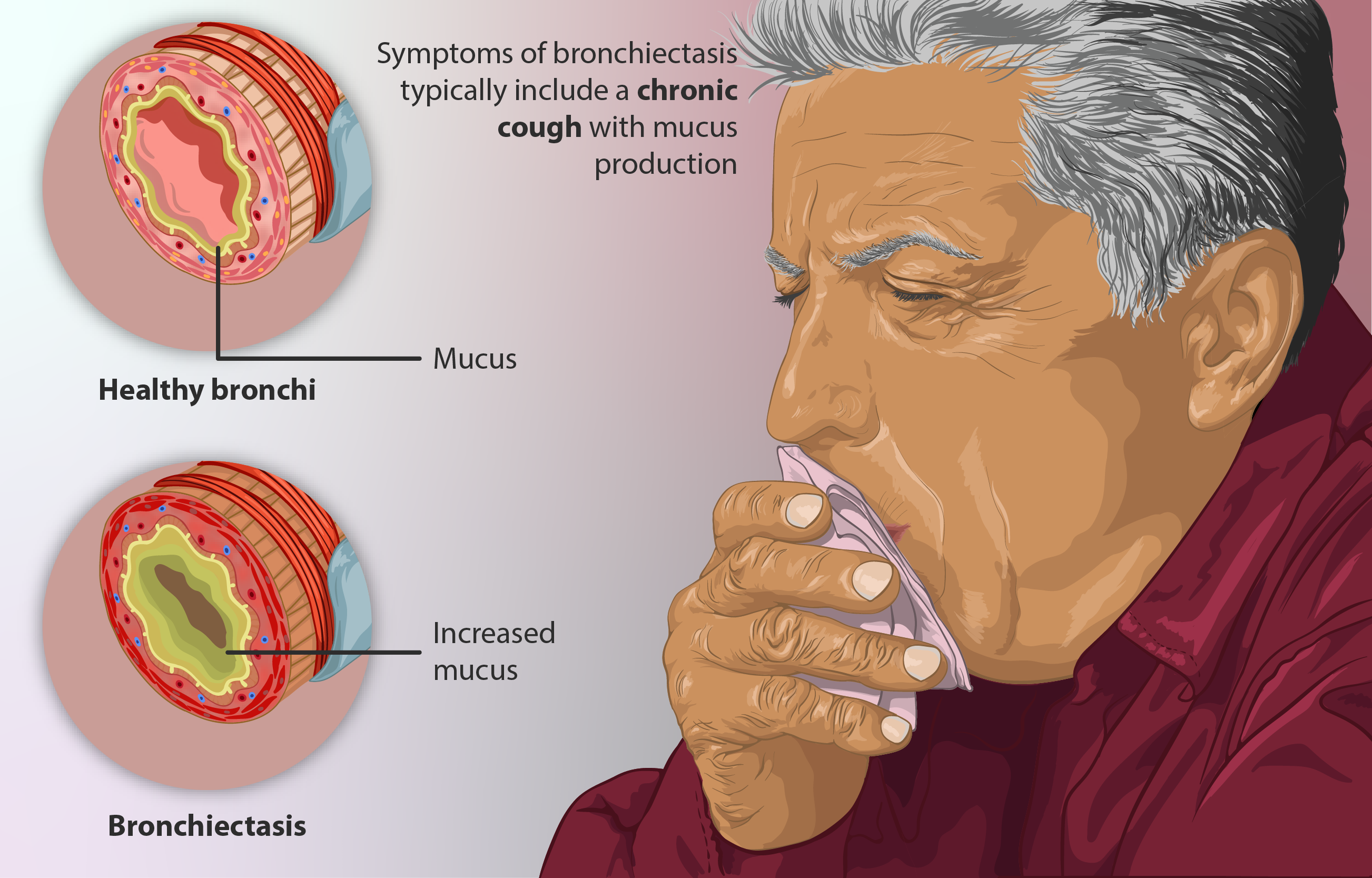
Non-Cystic Fibrosis Bronchiectasis and Chronic Cough Syndromes
Emerging data suggest potential benefits of inhaled ciclesonide in managing airway inflammation associated with chronic cough and non-CF bronchiectasis, although more robust studies are needed.
Eosinophilic Pneumonia & Allergic Bronchitis
Alvesco Inhaler COVID-19-Related Airway Inflammation and Post-Viral Cough
During the COVID-19 pandemic, inhaled corticosteroids like ciclesonide were explored for their anti-inflammatory effects on COVID-associated respiratory symptoms, including persistent post-infectious cough.
Seasonal Allergic Rhinitis via Intranasal Ciclesonide Formulation
An intranasal formulation of ciclesonide is used off-label for allergic rhinitis, capitalizing on its mucosal anti-inflammatory action with minimal systemic absorption.

6. Alvesco Dosage and Administration Guidelines
Alvesco Inhaler Dose on Prior Steroid Exposure
For steroid-naive patients, the usual starting dose is 80 mcg once daily. Patients previously maintained on inhaled or oral corticosteroids may begin at 160 mcg daily or higher as needed.
Titration for Symptom Control and Maximum Daily Limits
Doses may be titrated in increments of 80 mcg based on symptom persistence. The maximum recommended dose is 320 mcg once daily.
Step-Down Strategies in Well-Controlled Asthma
Upon sustained asthma control for several months, clinicians may reduce the dosage to the lowest effective dose, minimizing long-term corticosteroid exposure.
Missed Dose Protocols and Restart Considerations
If a dose is missed, patients are advised to take it as soon as remembered unless the next dose is near. Inhaler re-priming may be necessary if unused for over 10 days.
Transitioning from Other Inhaled Corticosteroids
When switching from another ICS, equivalent dosing should be calculated based on potency, and monitoring should be intensified during the first week of transition.
Alvesco Inhaler How to use
Take a breath while gently pushing the center of the dose indicator with your finger. Completely depress the canister until it no longer moves in the actuator while administering your dose. After inhaling the medication into your lungs through the inhaler device, hold your breath for 10 seconds or until it feels natural to exhale.
7. Handling Precautions and Proper Inhalation Technique
Device Priming, Shaking, and Actuation Steps
Before the first use, Alvesco should be primed by spraying three times into the air. It should be shaken gently and inhaled deeply through the mouth with each actuation.
Spacer or Holding-Chamber Compatibility
Alvesco is compatible with most valved holding chambers, especially beneficial for patients with poor inhalation coordination or pediatric users.
Mouthpiece Cleaning, Disinfection, and Maintenance
The mouthpiece should be cleaned weekly with a dry cloth. Avoid immersing in water or using solvents that may damage the device.
Dose Counter Use and Canister Replacement Timing
Alvesco features a built-in dose indicator. Patients should monitor the remaining doses and replace the inhaler once it reaches zero or earlier if malfunction occurs.
8. Storage and Stability Information
Recommended Temperature and Humidity Range
Store Alvesco at controlled room temperature between 15°C and 30°C. Avoid storage in environments with excessive humidity.
Protection from Heat, Freezing, and Direct Sunlight
As a pressurized inhaler, Alvesco should be protected from temperatures above 50°C and never frozen. Keep away from direct sunlight and heat sources.
Shelf-Life After First Actuation
After first use, the canister should be used within the period specified on the label, typically around 12 months, depending on frequency of administration.
Environmentally Safe Disposal of Pressurized Canisters
Do not puncture or incinerate the empty canister. Follow local regulations for aerosol disposal to minimize environmental impact.
9. Drug and Product Interactions
Ciclesonide is primarily metabolized by the hepatic enzyme CYP3A4. Concomitant use with strong inhibitors such as ketoconazole, ritonavir, itraconazole, and clarithromycin, may significantly increase systemic concentrations of its active metabolite, des-ciclesonide. This interaction heightens the risk of systemic corticosteroid effects, including adrenal suppression and Cushingoid features.
Combined Use with β2-Agonists, LABAs, and Leukotriene Modifiers
Alvesco is often prescribed alongside long-acting β2-agonists (LABAs) like salmeterol or formoterol and leukotriene receptor antagonists (LTRAs) such as montelukast. While these combinations can synergistically enhance asthma control, they also necessitate careful dose titration and regular evaluation to avoid overtreatment or masking of worsening symptoms.
Immunosuppressants and Systemic Corticosteroid Overlap
Simultaneous use with other immunosuppressants, such as methotrexate, cyclosporine, or oral corticosteroids, may compound the risk of infection, delayed wound healing, or systemic toxicity. Clinical judgment is essential to avoid excessive immunosuppression, particularly during prolonged treatment durations.
Vaccination Considerations
Although inhaled ciclesonide has limited systemic absorption, caution is advised with live attenuated vaccines (e.g., MMR, varicella). Immunocompromised states induced by high-dose inhaled or systemic corticosteroids may blunt vaccine efficacy or increase susceptibility to vaccine-derived infection. Inactivated vaccines remain safe and recommended.
10. Alvesco Inhaler Side Effects
Common Adverse Reactions
- Dysphonia and Oropharyngeal Candidiasis: Hoarseness and fungal infections of the mouth or throat are frequent due to local immunosuppression. Proper inhalation technique and mouth rinsing mitigate this risk.
- Headache, Nasopharyngitis, and Cough: These non-specific upper respiratory complaints are common but usually self-limiting and mild in intensity.
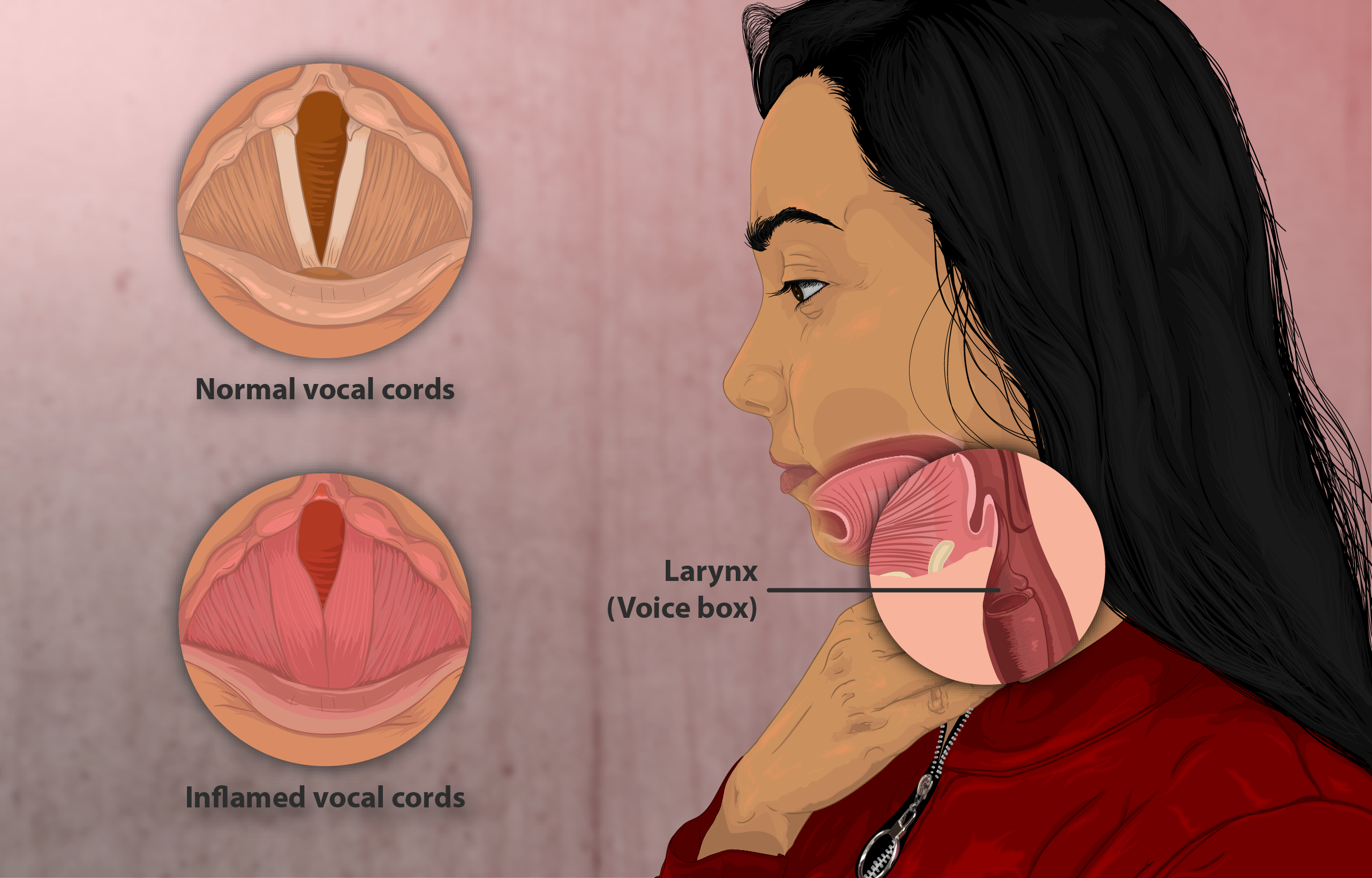
Less Common but Clinically Significant Effects
- Adrenal Suppression and HPA-Axis Effects: Prolonged high-dose exposure may suppress endogenous cortisol production, necessitating tapering and adrenal function monitoring during withdrawal.
- Ocular Complications Glaucoma & Cataracts: Long-term corticosteroid use, even via inhalation, may increase intraocular pressure or accelerate posterior subcapsular cataract formation. Regular ophthalmologic exams are prudent.
- Paradoxical Bronchospasm: Rare but potentially serious, this reaction requires immediate discontinuation and replacement with an alternative controller therapy.
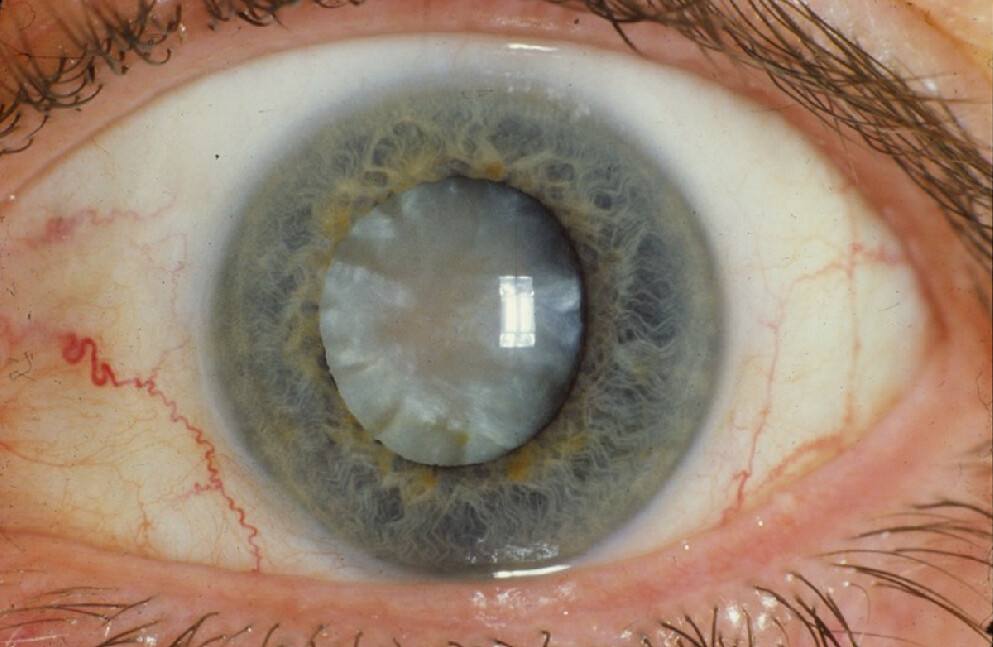
Long-Term Safety Monitoring Recommendations
Routine clinical surveillance should include:
- Height measurements in pediatric patients
- Intraocular pressure assessment in long-term users
- Morning serum cortisol in suspected adrenal suppression cases
- Lung function tests and symptom diaries for disease control evaluation
11. Contraindications
Hypersensitivity to Ciclesonide or HFA Propellant
Alvesco is contraindicated in individuals with known hypersensitivity to ciclesonide or hydrofluoroalkane components. Symptoms may include urticaria, bronchospasm, or angioedema.
Acute Status Asthmaticus Requiring Intensive Care
This formulation is not intended for the relief of acute bronchospasm or status asthmaticus. In such scenarios, rapid-acting bronchodilators and systemic corticosteroids are the standard of care.
Untreated Local or Systemic Fungal Infections
Inhaled corticosteroids may worsen fungal infections by impairing local immune defense. Alvesco should be avoided until adequate antifungal treatment is initiated.
12. Warnings and Important Precautions
Abrupt Withdrawal and Risk of Asthma Exacerbation
Discontinuing Alvesco suddenly can destabilize asthma control. Gradual tapering under medical supervision is essential, particularly in those transitioning from systemic steroids.
Systemic Corticosteroid Effects at Higher Doses
At high dosages or prolonged use, ciclesonide may exert systemic effects akin to oral corticosteroids manifesting as mood changes, hypertension, or glucose intolerance.
Immunosuppression and Delayed Wound Healing
Patients on Alvesco may have blunted immune responses, predisposing them to infections and slower wound healing, especially post-surgery or injury.
Impact on Linear Growth in Pediatric Patients
Growth velocity may be modestly reduced in children on chronic inhaled corticosteroids. Periodic height monitoring is recommended to detect early deviations.
Ocular Exam
Glaucoma screening is advisable in patients with personal or familial ocular disease risk, given the potential for corticosteroid-induced pressure elevation.
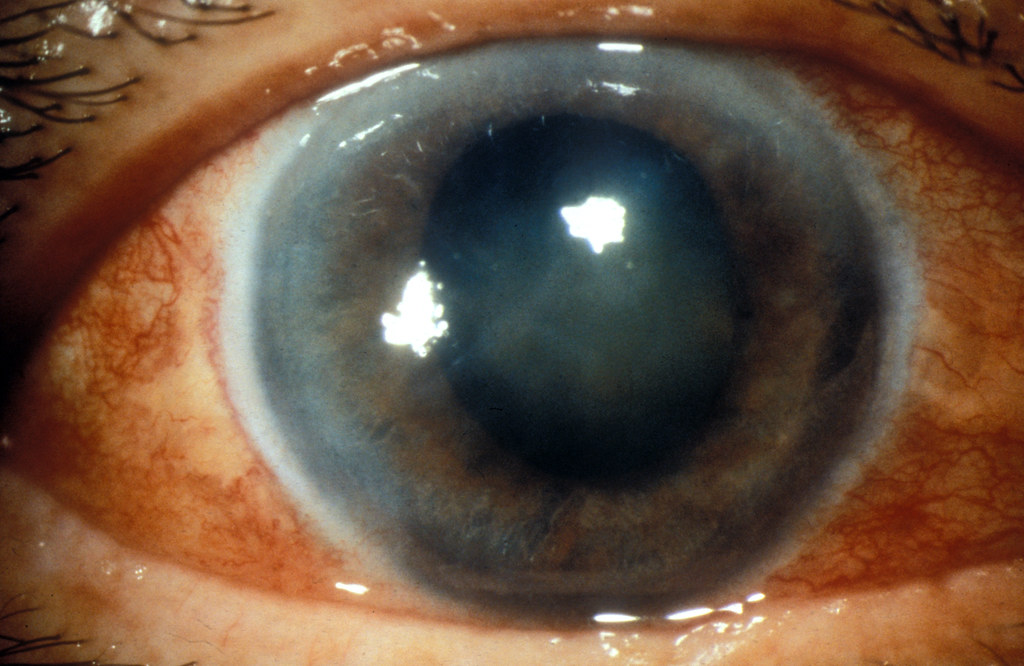
13. Careful Administration in Special Populations
Elderly Patients with Comorbid Cardiopulmonary Disease
Elderly individuals may have reduced pulmonary reserve or concurrent cardiac issues. Inhaler technique, fall risk, and comedication profiles should be closely monitored.
Pregnant Women: Risk Assessment and Lowest Effective Dose
Although animal studies have not shown teratogenicity at inhaled doses, Alvesco should be used during pregnancy only if clearly needed. The goal is to use the lowest dose that maintains asthma control.
Nursing Mothers: Breast-Milk Exposure Considerations
While systemic exposure is low, it is unknown if ciclesonide or its metabolites are excreted in human milk. Consideration should be given to the benefit of breastfeeding vs. the potential drug exposure.
Children and Adolescents: Growth and Adherence Monitoring
In pediatric populations, adherence challenges are common. Supervision of inhalation technique and behavioral reinforcement improve outcomes and mitigate growth-related risks.
Patients with Hepatic or Renal Impairment
Although inhaled drugs exert minimal systemic load, hepatic dysfunction may impair ciclesonide metabolism. Clinical monitoring is warranted in patients with moderate to severe hepatic impairment.
Athletes: Anti-Doping Compliance
Ciclesonide is permitted under WADA regulations when used via inhalation at approved doses. Documentation of therapeutic use exemption (TUE) may be required for competitive athletes.
14. Overdosage and Emergency Management
Clinical Signs of Acute Over-Inhalation
An acute overdose of Alvesco may present with symptoms resembling systemic steroid exposure: nausea, insomnia, facial puffiness, or mood alterations. However, such events are rare due to its local action and low bioavailability.
Immediate Supportive Measures and Observation
No specific antidote exists. Observation, symptomatic management, and discontinuation of excess doses are standard. Cardiovascular status and mental health should also be evaluated.
Long-Term Monitoring for Adrenal Insufficiency
In chronic overdose situations, patients should undergo endocrine testing to assess adrenal function. Morning serum cortisol and ACTH stimulation tests may be required to rule out suppression.
15. Patient Counseling and Adherence Strategies
Importance of Daily Maintenance vs. Rescue Inhalers
Patients should be educated that Alvesco is a preventive medication, not a reliever. Its regular use reduces exacerbation risk but will not provide immediate relief during asthma attacks.
Mouth Rinsing to Reduce Oral Thrush Risk
Rinsing the mouth with water and spitting after each dose is a simple but crucial strategy to prevent oropharyngeal candidiasis, particularly in long-term users.
Personalized Asthma Action Plans and Peak-Flow Monitoring
Developing a customized asthma action plan empowers patients to recognize early warning signs of worsening symptoms. Peak-flow meters can be used at home to track pulmonary function.
Digital Dose-Tracking Apps and Reminder Tools
Utilizing smartphone apps or smart inhaler systems helps improve adherence and prevent missed doses. These tools offer real-time reminders, data logging, and physician integration features.
Alvesco Inhaler FAQ
- What is an Alvesco inhaler used for?
- How often should I use Alvesco inhaler?
- Is Alvesco a preventer or reliever?
- Is Alvesco a cortisone steroid?
- Should I shake Alvesco before use?
- Who should not use Alvesco?
- How quickly does Alvesco work?
- Can I take 2 puffs of Alvesco?
- Can you take Alvesco long term?
- What happens if I stop taking my asthma inhaler?
- When is the best time to take Alvesco?
- How do you know when Alvesco is empty?
- Do you need to rinse after Alvesco?
- Can Alvesco cause heart palpitations?
- How fast does Alvesco work?
- Does Alvesco weaken your immune system?
- What age group is Alvesco for?
- What are the benefits of Alvesco?
- When was Alvesco approved?
- What does Alvesco do for your lungs?
- Is Alvesco inhaler a steroid?
- How to use Alvesco inhaler?
- How many puffs in Alvesco inhaler?
- What does an alvesco inhaler do?
What is an Alvesco inhaler used for?
Alvesco (also known as ciclesonide) is a steroid inhalation therapy commonly prescribed to manage asthma in individuals aged 12 years and above.
How often should I use Alvesco inhaler?
Twice a day
Is Alvesco a preventer or reliever?
Mono-therapy or single therapy preventer inhalers
Is Alvesco a cortisone steroid?
Alvesco includes the ingredient ciclesonide, classified as a corticosteroid medication.
Should I shake Alvesco before use?
No
Who should not use Alvesco?
People with allergic reaction fro Alvesco
How quickly does Alvesco work?
4 weeks or more
Can I take 2 puffs of Alvesco?
Yes
Can you take Alvesco long term?
Extended use of Alvesco may lead to a decrease in cortisol production by your adrenal glands.
What happens if I stop taking my asthma inhaler?
- Breathing problems may return
- Chest feels tight
- Shortness of breath
When is the best time to take Alvesco?
Alvesco is commonly taken two times a day, in the morning and in the evening.
How do you know when Alvesco is empty?
Remember to check the dose indicator display window after you've done the priming sprays before your use to confirm that it displays 60 sprays remaining.
Do you need to rinse after Alvesco?
Remember to rinse your mouth with water after taking medication to lower the chances of getting a mouth infection.
Can Alvesco cause heart palpitations?
Yes
How fast does Alvesco work?
Within 24 hrs
Does Alvesco weaken your immune system?
Yes
What age group is Alvesco for?
12 years of age and older
What are the benefits of Alvesco?
The maintenance inhaler is created to penetrate into the lungs and address issues in both large and small air passages effectively. Alvesco could potentially help individuals breathe more comfortably by reducing airway inflammation.
When was Alvesco approved?
Jan 16, 2008
What does Alvesco do for your lungs?
Alvesco acts as a corticosteroid that reduces airway inflammation in the lungs, enhancing airflow and facilitating breathing.
Is Alvesco inhaler a steroid?
Yes
How to use Alvesco inhaler?
Take a breath. Gently push the center of the dose indicator with your finger, ensuring the canister remains stationary in the inhaler during the dose administration process. Hold your breath for 10 seconds after inhaling or for as long as feels comfortable.
How many puffs in Alvesco inhaler?
60 puffs
What does an alvesco inhaler do?
Alvesco (also known as ciclesonide) is an inhalant containing steroids that is prescribed for managing asthma in adults and children aged 12 and above.