1. Comprehensive Introduction to Ivomec Injection
1.1 Definition and Therapeutic Category
Ivomec Injection is a broad-spectrum antiparasitic agent formulated for veterinary use. It belongs to the macrocyclic lactone class of endectocides, primarily used for the treatment and control of internal and external parasites in livestock.
1.2 Historical Development of Ivermectin Formulations
Originally derived from *Streptomyces avermitilis*, ivermectin was introduced in the 1980s and revolutionized antiparasitic therapy in both human and animal medicine. Ivomec was among the first injectable forms made available for veterinary use, rapidly becoming a cornerstone in parasite management programs.
1.3 Regulatory Approvals Across Regions (US, EU, Asia-Pacific, LATAM)
- United States: FDA-approved for cattle and swine under the Animal Drug Approval process.
- European Union: EMA classifies it under veterinary medicinal products, with country-specific labeling.
- Asia-Pacific: Widely licensed in Australia, India, and Southeast Asia for livestock use.
- Latin America: Commonly approved across Brazil, Argentina, and Chile, often under local brand equivalents.
1.4 Market Availability: Single-Dose Vials vs. Multidose Containers
Ivomec is commercially available in sterile glass or plastic containers, ranging from 1 mL unit-dose syringes to 500 mL multidose vials, enabling flexibility for use in both small farms and large-scale commercial operations.
2. Chemical Composition and Pharmaceutical Formulation
2.1 Active Ingredient: Ivermectin Concentration per mL
Each milliliter of Ivomec Injection contains 10 mg of ivermectin, providing a standardized dosage across formulations to ensure consistent therapeutic efficacy.
2.2 Inactive Excipients and Their Functional Roles
- Glycerol formal: Solubilizes ivermectin and stabilizes the active ingredient.
- Propylene glycol: Aids in viscosity and prevents microbial growth.
2.3 Physicochemical Properties Affecting Stability and Potency
The solution is clear, slightly viscous, and light-sensitive. Its lipid solubility enhances absorption, while the formulation is optimized for prolonged tissue retention.
2.4 Packaging Materials and Sterility Assurance Measures
Packaging is designed to maintain sterility, typically featuring rubber stoppers and crimped aluminum seals. Multidose containers often include preservatives to prevent contamination upon repeated entry.
3. Mechanism of Action: How Ivomec Injection Works
3.1 Binding to Glutamate-Gated Chloride Channels in Parasites
Ivomec selectively binds to glutamate-gated chloride ion channels in the nerve and muscle cells of parasites, increasing permeability to chloride ions and disrupting neural transmission.
3.2 Paralysis and Death of Endo- and Ectoparasites
This disruption results in irreversible paralysis, leading to death of the parasite. The mechanism is lethal to both endoparasites like nematodes and ectoparasites such as mites and lice.
3.3 Spectrum of Activity: Nematodes, Arthropods, and Off-Target Organisms
Effective against gastrointestinal worms, lungworms, mites, ticks, and lice. Its spectrum excludes cestodes and trematodes due to their different neuroreceptor composition.
3.4 Resistance Mechanisms and Genetic Markers in Target Species
Resistance is emerging via mutations in P-glycoprotein and β-tubulin genes. Rotational use of anthelmintics is recommended to mitigate this risk.
4. Approved Veterinary Uses and Indications
4.1 Cattle: Gastrointestinal Roundworms, Lungworms, Warbles, Lice, Mange Mites
Ivomec provides robust protection against adult and larval stages of parasites common in bovines. It also targets Hypoderma larvae responsible for warbles.
4.2 Swine: GI Roundworms, Lungworms, Kidney Worms, Lice, Mange
Recommended for routine swine deworming programs, especially for Ascaris suum, lungworms, and sarcoptic mange infestations.
4.3 Sheep and Goats: Nasal Bots, Psoroptic Mange, GI Parasites
Ivomec controls both adult and immature stages of GI nematodes and is also effective against nasal bot fly larvae (*Oestrus ovis*).
4.4 Reindeer and Deer: Warble Larvae and Nasal Bots
Used in controlled wildlife and managed cervid populations to prevent infestations from botflies and warbles, improving overall herd health.
4.5 Camelids and Other Livestock: Emerging Label Extensions
Though off-label in many regions, it is used under veterinary guidance for llamas, alpacas, and buffalo to control strongyles and skin parasites.
5. Off-Label and Extra-Label Applications
5.1 Companion Animals (Dogs, Cats) for Demodicosis and Ear Mites
Used cautiously in canines with demodectic mange and *Otodectes cynotis* infestations. Breed sensitivity (e.g., Collies) must be accounted for.
5.2 Equines for Onchocerciasis and Midge-Borne Parasites
Occasionally employed for *Onchocerca cervicalis*, though not a licensed equine product. Veterinary discretion is critical due to dosage sensitivity.
5.3 Wildlife and Zoo Species: Parasite Management Protocols
Formulated regimens in exotic mammals and birds have been reported, especially in zoological settings. Monitoring and individualized dosing are imperative.
5.4 Aquaculture and Apiculture: Investigational Anti-Parasitic Uses
Studies have explored ivermectin’s utility against sea lice in salmon and *Varroa destructor* in honeybees, though concerns over toxicity and residues persist.
5.5 Human Medicine Controversies and Public Health Guidance
Despite media attention during pandemics, health agencies such as the FDA and WHO advise against veterinary ivermectin use in humans outside of approved clinical trials.
6. Dosage and Administration Guidelines
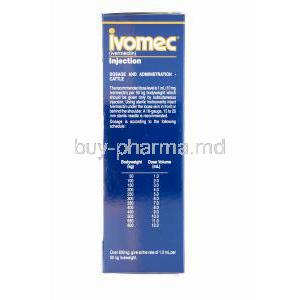
6.1 Standard Dose Rates by Species and Body Weight
- Cattle: 200 mcg/kg body weight (1 mL per 50 kg)
- Swine: 300 mcg/kg body weight (1 mL per 33 kg)
- Sheep and Goats: 200 mcg/kg body weight, under veterinary advice
6.2 Route of Injection: Subcutaneous vs. Intramuscular Techniques
Primarily administered via subcutaneous injection in the neck. Intramuscular use is not typically recommended due to tissue irritation risk.
6.3 Treatment Schedules: Single-Dose, Strategic Deworming, Seasonal Control
Usage depends on parasite load, region, and farming practice. Many producers adopt spring and fall treatments for effective parasite suppression.
6.4 Meat, Milk, and Slaughter Withdrawal Periods
Withdrawal times vary by species and jurisdiction. Typically:
- Meat: 35-49 days (depending on region)
- Milk: Not approved for lactating dairy cattle
6.5 Step-by-Step Administering Instructions for Field Technicians
- Weigh the animal or estimate body weight accurately.
- Disinfect the injection site.
- Draw the correct volume using sterile technique.
- Inject subcutaneously into the neck region.
- Record batch number and date of administration.
7. Handling Precautions and Safe Use Protocols
7.1 Personal Protective Equipment (PPE) Recommendations
Wear gloves and protective eyewear to avoid dermal or ocular exposure. Wash hands thoroughly after handling.
7.2 Needle Hygiene and Injection-Site Selection
Use a sterile needle for each animal to prevent cross-contamination. Rotate injection sites to avoid tissue damage.
7.3 Environmental Safety: Protecting Waterways and Beneficial Insects
Avoid application near aquatic systems. Ivermectin is highly toxic to fish and dung beetles, contributing to ecological disruption.
7.4 Proper Disposal of Unused Product and Sharps
Follow local regulations for hazardous waste. Used needles should be placed in puncture-resistant containers and discarded at approved facilities.
8. Storage, Shelf Life, and Transport Requirements
8.1 Optimal Temperature Range and Light Protection
Store between 15°C and 30°C. Protect from light and do not refrigerate or freeze, as this may precipitate active ingredients.
8.2 Stability After First Puncture: In-Use Period Guidelines
Once opened, use the product within 28 days unless otherwise specified by the manufacturer. Label the vial with the date of first use.
8.3 Transportation in High-Temperature Climates
Use insulated containers or cold packs if transport exceeds 30°C. Avoid prolonged exposure to direct sunlight.
8.4 Signs of Product Degradation and When to Discard
Discard if the solution appears cloudy, discolored, or precipitated. Follow disposal guidelines to prevent environmental contamination.
9. Drug and Chemical Interactions
9.1 Synergistic or Additive Antiparasitic Combinations
Ivomec Injection may be used alongside other antiparasitic agents to enhance therapeutic efficacy. Synergistic combinations, such as ivermectin with clorsulon or praziquantel, broaden the spectrum of parasite coverage by targeting different classes like trematodes and cestodes. However, such use should be based on informed veterinary judgment to avoid cumulative toxicity.
9.2 Contraindicated Drugs Affecting P-Glycoprotein Efflux
Drugs that inhibit P-glycoprotein (P-gp), a cellular efflux transporter, can elevate ivermectin levels in the central nervous system. This includes agents like ketoconazole, cyclosporine, and verapamil. In animals with a mutated MDR1 gene (e.g., some herding breeds), these interactions can increase neurotoxicity risk, leading to seizures or coma.
9.3 Impact of Corticosteroids, Sedatives, or Vaccines
- Corticosteroids: May mask early symptoms of toxicity and modulate immune responses, potentially delaying parasite clearance.
- Sedatives: Co-administration may enhance central nervous system depression in sensitive species.
- Vaccines: Anthelmintic treatment should be spaced to avoid immunosuppressive overlap, particularly in stressed animals.
9.4 Feed Additives and Mineral Interference
While not directly reactive, certain feed additives, such as high-fat carriers, may alter absorption kinetics of orally administered macrocyclic lactones. Injectable formulations like Ivomec are less affected but still warrant attention during combined administration with mineral boluses or medicated feeds.
10. Contraindications and Populations Requiring Careful Administration
10.1 Known Breed Sensitivities (e.g., MDR1-Mutant Collies)
Breeds like Collies, Australian Shepherds, and Shetland Sheepdogs may carry a genetic mutation in the MDR1 gene, impairing their ability to expel ivermectin from the CNS. Even standard doses can trigger neurotoxic effects such as tremors, disorientation, or coma. Genetic testing prior to administration is strongly advised.
10.2 Severe Hepatic or Renal Impairment in Target Animals
Animals with compromised hepatic or renal function may exhibit reduced drug clearance, increasing the risk of drug accumulation and systemic toxicity. Ivomec should be avoided or used cautiously with adjusted dosing under close supervision.
10.3 Concurrent Disease States Compromising Safety
Infections, malnutrition, or concurrent parasitism can exacerbate drug effects or reduce tolerance. Immunocompromised animals may have unpredictable responses to antiparasitic agents, necessitating tailored therapeutic strategies.
10.4 Situations Warranting Dose Adjustment or Alternatives
- Neonates with underdeveloped metabolic pathways
- Heavily parasitized animals prone to die-off shock
- Off-label species requiring careful titration
11. Warnings and Critical Safety Notices
11.1 Neurologic Toxicity Signs and Emergency Response
Signs include mydriasis, ataxia, head pressing, and respiratory depression. Emergency response involves supportive care, oxygenation, and in severe cases, mechanical ventilation. There is no specific antidote for ivermectin toxicity.
11.2 Accidental Self-Injection Risks for Handlers
Humans should avoid direct exposure. In case of accidental self-injection, immediate medical consultation is necessary, particularly if symptoms like dizziness, nausea, or numbness occur. Proper training and the use of safety-engineered syringes are essential.
11.3 Legal Restrictions on Extra-Label Use in Food Animals
Extra-label use is governed by national regulations such as the AMDUCA (Animal Medicinal Drug Use Clarification Act) in the U.S. Non-compliance may lead to residue violations, legal penalties, or withdrawal of marketing authorization.
11.4 Environmental Impact Statements and Wildlife Hazards
Ivermectin residues in dung can harm dung beetles and other beneficial insects, affecting pasture ecosystems. Care should be taken to avoid runoff into aquatic environments, as it is highly toxic to fish and amphibians.
12. Common and Less Common Side Effects
12.1 Local Reactions: Swelling, Tenderness, Abscess Formation
Localized irritation at the injection site may occur. Although typically self-limiting, abscess formation can result from non-sterile technique or product contamination. Avoid injecting more than 10 mL at a single site in large animals.
12.2 Systemic Reactions: Lethargy, Ataxia, Hypersalivation
These effects are rare at therapeutic doses but may appear in sensitive animals or following overdose. Observation for 24–48 hours post-treatment is recommended in high-risk groups.
12.3 Hypersensitivity-Like Reactions from Rapid Parasite Kill
Massive parasite die-off can provoke a systemic inflammatory response resembling an allergic reaction. Symptoms include pyrexia, tachycardia, and labored breathing, requiring prompt veterinary intervention.
12.4 Post-Treatment Monitoring and Reporting Protocols
Practices should implement monitoring plans to detect and document adverse reactions. Reporting systems like the FDA’s CVM (Center for Veterinary Medicine) adverse event reporting can enhance pharmacovigilance.
13. Important Precautions in Special Populations
13.1 Administration to Elderly or Geriatric Animals
Aging animals often have decreased organ function and may metabolize drugs more slowly. Regular monitoring of renal and hepatic parameters is advisable during antiparasitic treatment in geriatric patients.
13.2 Use in Pregnant Animals: Teratogenicity and Fetal Safety Data
Studies in several species show no significant teratogenicity at recommended doses. However, high doses or off-label use should be avoided during the first trimester unless benefits outweigh risks.
13.3 Injection During Lactation: Milk Residue Considerations
Ivomec is not approved for use in lactating dairy cattle. Residues can persist in milk, posing food safety concerns. Milk discard times should be strictly observed where off-label use is necessary.
13.4 Pediatric Use: Neonates, Weanlings, and Growing Stock
Young animals may be more susceptible to central nervous system side effects due to immature blood-brain barrier development. Pediatric use should follow species-specific guidelines with adjusted dosages and close observation.
14. Over-Dosage and Toxicity Management
14.1 Recognizing Clinical Signs of Ivermectin Overexposure
- Muscle tremors
- Unsteady gait
- Hypothermia
- Seizures or coma in extreme cases
14.2 First-Aid Measures and Veterinary Interventions
Immediate veterinary evaluation is critical. Induce emesis in cases of oral ingestion (if safe), administer activated charcoal, and initiate fluid therapy to support renal excretion.
14.3 Supportive Care Protocols and Prognosis
Supportive care may involve intravenous fluids, nutritional support, and anticonvulsants. Prognosis is generally favorable with early intervention, but prolonged coma can lead to permanent deficits or fatality.
14.4 Preventive Strategies to Avoid Accidental Overdosing
Always verify species-specific dose ranges and calibrate dosing devices. Use color-coded syringes or dose charts to prevent human error. Training and refresher sessions for animal handlers are highly recommended.
15. Careful Administration and Risk Mitigation Strategies
15.1 Split-Dosing Large Animals to Reduce Injection-Site Volume
Large-volume injections can lead to tissue necrosis. Divide doses over multiple sites and limit each to 10 mL or less to minimize adverse reactions.
15.2 Rotation with Other Anthelmintic Classes to Delay Resistance
Alternating ivermectin with benzimidazoles, levamisole, or moxidectin helps reduce the emergence of resistance. Strategic rotation should be guided by fecal egg count reduction testing (FECRT).
15.3 Integrating Fecal Egg Count Monitoring Programs
Routine fecal sampling enables targeted treatment and reduces unnecessary drug use. This supports sustainable parasite control and delays resistance development.
15.4 Educating Farm Personnel on Label Compliance
Clear training materials and label interpretation workshops empower farm staff to administer medications responsibly. Emphasizing withdrawal periods and proper injection techniques ensures compliance and animal welfare.
16. Handling Precautions During Mass Treatment Campaigns
16.1 Biosecurity Measures When Treating Multiple Herds
Use dedicated equipment for each group or thoroughly disinfect between uses. Avoid moving between pens without protective clothing changes to limit cross-contamination.
16.2 Cold-Chain Maintenance in Remote Settings
Ensure storage between 15°C and 30°C during field deployments. Use insulated containers and monitor temperature with data loggers to maintain drug efficacy.
16.3 Record-Keeping for Traceability and Audit Compliance
Maintain batch numbers, administration dates, dosages, and operator identification. Digital herd health management systems streamline traceability and meet regulatory audit requirements.
16.4 Community Outreach on Responsible Anthelmintic Use
Educational campaigns targeting smallholders and pastoral communities can improve understanding of parasite control strategies, reducing misuse and environmental contamination. Public-private partnerships may enhance outreach effectiveness.