Introduction to Susten Vaginal Gel
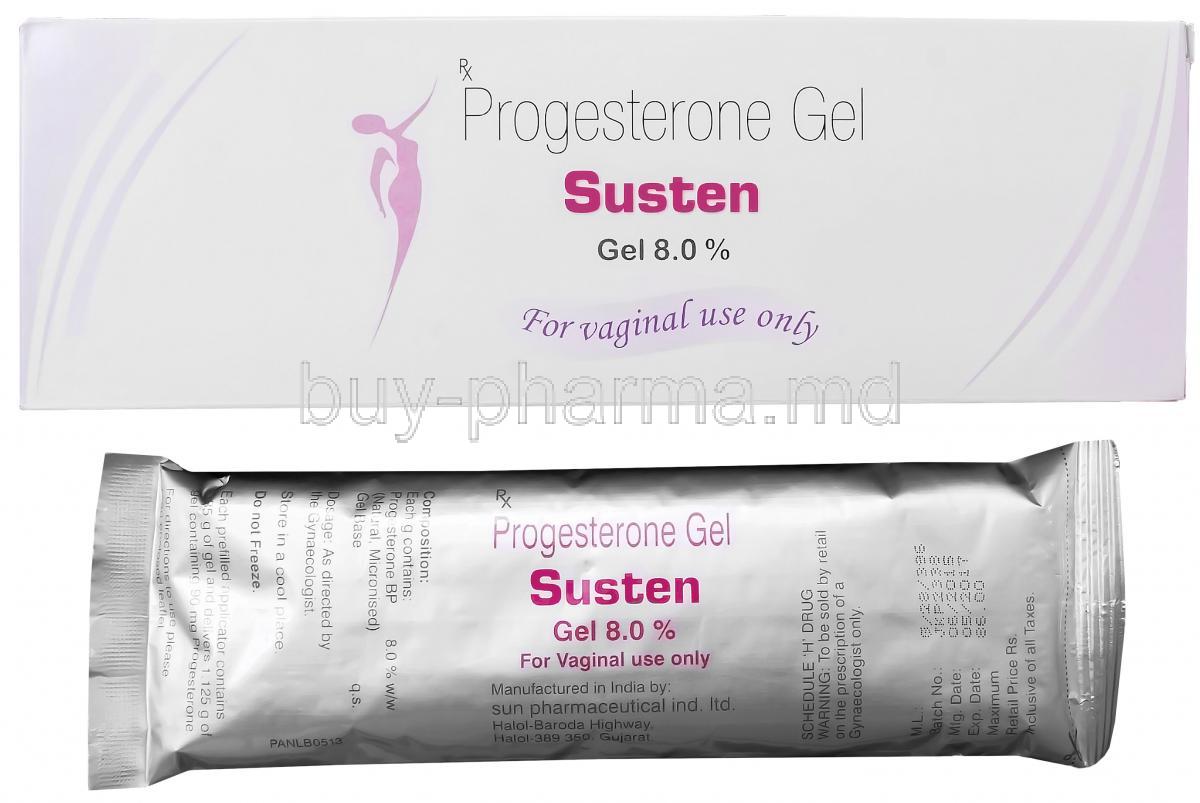
Susten Vaginal Gel is a specialized progesterone-based formulation designed to support reproductive health, particularly in women undergoing fertility treatments. Classified as a natural micronized progesterone product, it plays a pivotal role in gynecological and assisted reproductive practices.
This vaginal gel is widely employed to provide luteal phase support, a critical phase in the menstrual cycle that ensures endometrial receptivity for embryo implantation. Susten is available in convenient, pre-filled applicators with commonly used strengths such as 8% (90 mg per applicator), enabling precise and hygienic administration.
Compared to oral or injectable progesterone, Susten Vaginal Gel offers a targeted delivery system with improved uterine bioavailability and fewer systemic side effects, making it a preferred option in ART protocols.
Pharmacological Action: How Susten Vaginal Gel Works
Susten Vaginal Gel delivers natural micronized progesterone directly into the vaginal canal, from where it is rapidly absorbed into the systemic and uterine circulation. Progesterone is vital for transforming the endometrium into a secretory lining that can support embryo implantation and early pregnancy development.
- Facilitates luteal phase support by stabilizing the endometrium
- Enhances embryo implantation success in IVF and IUI procedures
- Maintains pregnancy by preventing premature shedding of the uterine lining
The vaginal route bypasses hepatic first-pass metabolism, offering enhanced local bioavailability and reducing the burden on the liver.
Composition and Formulation Details
Each gram of Susten Vaginal Gel typically contains 8% natural micronized progesterone (equivalent to 90 mg per 1.125 g applicator). The gel base consists of bioadhesive, water-soluble polymers that ensure sustained contact with the vaginal mucosa.
- Active Ingredient: Natural Micronized Progesterone
- Excipients: Polycarbophil, glycerin, carbomer, sodium hydroxide, purified water
- pH and Osmolarity: Formulated to match vaginal physiological conditions
- Preservatives: Free from parabens and artificial preservatives
Approved Medical Uses of Susten Vaginal Gel
Susten Vaginal Gel is approved for several hormone-related therapeutic applications, primarily in women undergoing fertility treatments or experiencing hormonal imbalances:
- Luteal phase support during in vitro fertilization (IVF) or intrauterine insemination (IUI)
- Progesterone supplementation in women diagnosed with luteal phase defect
- Adjunct in hormone replacement therapy for menopausal and perimenopausal symptoms
- Prevention of endometrial hyperplasia in women receiving estrogen therapy
Off-label and Investigational Uses
Beyond approved uses, Susten Vaginal Gel is also used in several investigational and off-label scenarios, particularly in the field of reproductive endocrinology:
- Management of recurrent miscarriage linked to inadequate luteal support
- Supportive therapy in threatened miscarriage during early gestation
- Empirical use in women with unexplained infertility or irregular cycles
- Progesterone support in anovulatory cycles related to polycystic ovary syndrome (PCOS)
Dosage Guidelines and Administration Instructions
The recommended dosage of Susten Vaginal Gel varies based on indication and fertility protocol. In IVF protocols, a common regimen includes one applicator (90 mg) administered vaginally once or twice daily, starting from the day of embryo transfer and continued up to 10-12 weeks of gestation if pregnancy occurs.
For natural or stimulated cycles, dosage may differ and must be guided by a healthcare provider. The gel is applied using the pre-filled applicator, inserted into the vagina in a reclined position.
- Do not skip doses—adherence ensures therapeutic progesterone levels
- In case of missed dose, apply it as soon as remembered unless it is near the time for the next dose
Potential Side Effects and Adverse Reactions
7.1 Common Side Effects of Susten Vaginal Gel
- Localized vaginal irritation, discharge, or itching
- Breast tenderness, abdominal bloating
- Fatigue, mood swings, or mild headaches
- Transient abdominal discomfort or cramps
7.2 Serious and Less Common Side Effects
- Breakthrough vaginal bleeding or spotting
- Allergic reactions, including rash, pruritus, or swelling
- Severe migraine or visual disturbances (report immediately)
- Thromboembolic events—rare but possible with prolonged hormonal therapy
Drug and Substance Interactions
Susten Vaginal Gel may interact with certain drugs, particularly those influencing hormone metabolism:
- Combined estrogen therapy: Requires careful monitoring in HRT regimens
- CYP3A4 inducers (e.g., rifampin, carbamazepine): May reduce progesterone levels
- CYP3A4 inhibitors (e.g., ketoconazole): May increase systemic progesterone exposure
- Intravaginal antifungals or antibiotics: Potential to alter gel absorption or retention
Warnings and Important Safety Information
- Susten Vaginal Gel is not a contraceptive and does not prevent ovulation or pregnancy
- Should be used only under the supervision of a qualified healthcare professional
- Not recommended for women with current or past breast, uterine, or ovarian malignancies
- Caution advised in patients with a history of thromboembolic disorders, such as deep vein thrombosis or pulmonary embolism
Contraindications for Use
Susten Vaginal Gel should not be used in individuals with specific medical conditions or sensitivities due to the risk of adverse outcomes. Prior to initiating therapy, a thorough medical evaluation is essential.
- Hypersensitivity: Contraindicated in patients with known allergy to progesterone or any component of the gel formulation, which may result in local or systemic allergic reactions.
- Undiagnosed Vaginal Bleeding: Any abnormal or unexplained vaginal bleeding must be investigated prior to initiation, as it may signify a serious underlying pathology.
- Hormone-Sensitive Malignancies: Use is contraindicated in individuals with current or suspected breast, uterine, or ovarian cancers due to the potential for hormonal stimulation of malignant cells.
- Thromboembolic Disorders: Patients with a history of deep vein thrombosis (DVT), pulmonary embolism (PE), or other thrombotic events should not use progesterone due to increased clotting risk.
Precautions and Careful Administration
While Susten Vaginal Gel is generally well-tolerated, careful monitoring is required during prolonged or high-dose use to mitigate risks and optimize therapeutic outcomes.
- Hormonal Monitoring: Regular evaluation of endometrial thickness and serum hormone levels is advisable to assess response to therapy and prevent endometrial overstimulation.
- Pelvic Examinations: Long-term users should undergo routine pelvic assessments to detect any changes in uterine or ovarian structures.
- Organ Dysfunction: Dose modification may be necessary in patients with hepatic or renal impairment to prevent systemic accumulation of progesterone.
- Contraindicated Pregnancy Scenarios: Should be avoided in cases of incomplete abortion or suspected ectopic pregnancy due to safety concerns.
Special Populations: Use in Specific Groups
12.1 Use in Elderly Patients
Susten Vaginal Gel is not typically indicated for postmenopausal women unless part of a carefully supervised hormone replacement therapy regimen.
- Increased risk of systemic absorption in elderly with thin vaginal epithelium
- Consideration of comorbid conditions such as cardiovascular disease, hypertension, and hormone-sensitive cancers
12.2 Use in Pregnant and Breastfeeding Women
Susten Vaginal Gel is commonly used during the early stages of pregnancy, particularly in assisted reproductive techniques (ART) such as IVF, where progesterone support is essential.
- Pregnancy: Considered safe for use during the first trimester under medical supervision to support implantation and placental development
- High-Risk Pregnancies: May be used in women with a history of recurrent miscarriages or luteal phase defects, with ongoing gynecologic monitoring
- Breastfeeding: Small amounts of progesterone may be excreted into breast milk; clinical judgment is necessary when prescribing during lactation
12.3 Pediatric and Adolescent Administration
There is no established indication for the use of Susten Vaginal Gel in pediatric or adolescent females. Safety and efficacy data in this population are lacking.
- Not recommended for individuals under 18 years of age
- Should not be used for puberty-related hormonal disturbances
Overdose and Emergency Management
Though rare, overdose of progesterone through vaginal gel application can lead to symptomatic hormonal excess. Prompt recognition and appropriate response are crucial.
- Symptoms: May include fatigue, dizziness, nausea, breast pain, somnolence, or mood alterations
- Action: Discontinue application immediately upon suspected overdose
- Treatment: Supportive and symptomatic management; no specific antidote exists
- Prevention: Emphasize strict adherence to prescribed dosage and schedule
Storage and Handling Precautions
To maintain the efficacy and safety of Susten Vaginal Gel, proper storage and handling practices must be followed throughout the treatment duration.
- Store at controlled room temperature, typically below 25°C (77°F)
- Avoid exposure to direct sunlight, moisture, and excessive heat
- Check expiration date prior to use and discard any expired product
- Used applicators should be disposed of in accordance with local regulations; do not reuse
- During travel, maintain a stable temperature and carry in original packaging to prevent leakage or contamination
Handling Instructions and Patient Education
Correct administration and hygiene practices are essential to maximize the benefits of Susten Vaginal Gel and minimize potential complications.
- Wash hands thoroughly before and after application
- Use the pre-filled applicator as directed, preferably in a reclined or lying-down position for optimal absorption
- Do not skip doses; follow the schedule provided by the healthcare provider strictly
- If irritation, bleeding, or unusual symptoms occur, discontinue use and consult a physician promptly
- Educate patients on recognizing signs of allergic reaction or overdose