Introduction
Overview of Tiniba (Tinidazole) as an Antiprotozoal and Antibacterial Medication
Tiniba, containing the active compound tinidazole, is a potent antiprotozoal and antibacterial drug belonging to the nitroimidazole class. It is widely prescribed for infections caused by protozoa and anaerobic bacteria. With a broad spectrum of action, it targets some of the most persistent gastrointestinal and urogenital infections.
History and Development of Tinidazole
Tinidazole was developed as a second-generation nitroimidazole, designed to overcome limitations of earlier agents. Approved in multiple countries, it gained recognition due to its prolonged half-life and simplified dosing regimen, which enhances patient compliance compared to older therapies.
Comparison with Other Nitroimidazole Drugs
- Metronidazole: Effective but requires frequent dosing.
- Ornidazole: Similar mechanism, slightly longer duration of action.
- Secnidazole: Offers single-dose therapy for some infections but less widely available.
Tinidazole stands as a balanced option, combining efficacy, tolerability, and convenience.
Global Availability and Brand Names
Globally, tinidazole is marketed under numerous brand names, with Tiniba being one of the most recognized. It is available across Asia, Africa, Europe, and South America, and widely distributed in both hospital and retail pharmacy settings.
Composition of Tiniba (Tinidazole)
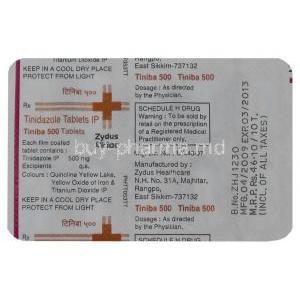
Active Ingredient: Tinidazole
The therapeutic activity derives entirely from tinidazole, a synthetic derivative of 5-nitroimidazole compounds.
Available Strengths and Dosage Forms
- Tablets: 300 mg, 500 mg, and 1,000 mg
- Oral suspension: designed for pediatric use
- Intravenous formulations: available in certain clinical settings
Inactive Ingredients and Excipients
Formulations may contain stabilizers, binders such as microcrystalline cellulose, and disintegrants to ensure proper absorption. Excipients vary depending on the manufacturer.
Mechanism of Action: How Tinidazole Works
Nitroimidazole Class Mechanism of Action
Tinidazole enters microbial cells and undergoes reductive activation, forming reactive metabolites. These metabolites disrupt critical cellular functions.
Inhibition of DNA Synthesis
The reactive intermediates generated bind to microbial DNA, inhibiting nucleic acid synthesis and leading to cell death. This explains its broad antimicrobial spectrum.
Selectivity for Anaerobic Organisms
Only anaerobic bacteria and protozoa possess the enzymatic machinery necessary for activation of tinidazole, sparing aerobic cells and human tissues from significant toxicity.
Pharmacokinetics
- Absorption: Rapid and nearly complete after oral intake
- Metabolism: Primarily hepatic via oxidation and conjugation
- Half-life: Approximately 12–14 hours
- Elimination: Excreted through urine and bile
Uses of Tiniba (Tinidazole)
FDA-Approved and Primary Indications
- Giardiasis: Effective against Giardia lamblia infections
- Amebiasis: Treats both intestinal disease and hepatic abscesses
- Trichomoniasis: Single-dose cure for Trichomonas vaginalis
- Bacterial Vaginosis: Restores vaginal flora balance
- Anaerobic Bacterial Infections: Intra-abdominal, gynecological, and respiratory infections
Off-Label Uses
- Prophylaxis in colorectal surgery to prevent postoperative infections
- Treatment of mixed protozoal infections
- Resistant or recurrent parasitic infections requiring extended regimens
- Investigational role in Helicobacter pylori eradication protocols
Dosage and Administration of Tiniba
General Dosing Guidelines for Adults
Tinidazole is typically prescribed in single or short-course regimens due to its long half-life. Dosages vary based on infection type.
Recommended Dosages by Condition
- Giardiasis: 2 g orally, single dose
- Amebiasis: 2 g daily for 3 days
- Trichomoniasis: 2 g orally, single dose
- Bacterial vaginosis: 2 g daily for 2 days or 1 g daily for 5 days
Pediatric Dosage and Considerations
Dosage is weight-based, typically 50 mg/kg, not to exceed 2 g per day. Pediatric suspension formulations improve administration in children.
Duration of Therapy
Most infections respond to 1–3 days of therapy, offering advantages over longer antibiotic courses.
Missed Dose and Overdose Management
Missed doses should be taken promptly unless near the next scheduled dose. Overdose may lead to neurological disturbances, necessitating supportive care.
Oral vs. Intravenous Administration
Oral therapy remains standard; IV formulations are reserved for patients unable to take medications orally or with severe systemic infections.
Side Effects of Tiniba (Tinidazole)
Common Side Effects
- Gastrointestinal upset: nausea, vomiting, abdominal discomfort, metallic taste
- CNS symptoms: headache, dizziness, mild fatigue
- Dermatological issues: rash, itching, flushing
Serious and Rare Side Effects
- Peripheral neuropathy with prolonged use
- Seizures and convulsions in rare cases
- Blood disorders: leukopenia, neutropenia
- Severe allergic reactions: angioedema, anaphylaxis
- Liver toxicity with abnormal liver function tests
Drug Interactions with Tiniba
- Alcohol: May cause a disulfiram-like reaction with flushing, nausea, and palpitations
- Anticoagulants: Potentiates effects of warfarin, requiring INR monitoring
- Lithium: Reduces clearance, leading to possible lithium toxicity
- CYP450 Pathways: Metabolic involvement increases risk of interactions with hepatic enzyme modulators
- Other Nitroimidazoles: Concomitant use may increase toxicity without enhancing efficacy
Contraindications of Tiniba
- Hypersensitivity to tinidazole or other nitroimidazole derivatives
- First trimester of pregnancy due to teratogenic potential
- History of blood dyscrasias such as aplastic anemia or agranulocytosis
- Existing neurological disorders that may worsen with therapy
Warnings and Important Precautions
Risk of Neurological Adverse Reactions
Tinidazole may provoke neurological disturbances, particularly with prolonged use or high doses. Patients can experience peripheral neuropathy, dizziness, or even seizures. Caution is imperative in individuals with a history of neurological disorders, and any emerging symptoms such as tingling, numbness, or motor weakness should prompt discontinuation.
Potential Mutagenic and Carcinogenic Effects from Animal Studies
Animal experiments have indicated possible mutagenic and carcinogenic effects with nitroimidazole derivatives. While human evidence remains inconclusive, healthcare providers generally recommend avoiding unnecessary or repeated courses of therapy unless clinically justified.
Avoid Alcohol During and After Treatment
Concomitant use of alcohol can trigger a disulfiram-like reaction, leading to flushing, abdominal cramps, tachycardia, nausea, and vomiting. Patients should abstain from alcohol during therapy and for at least 72 hours after completing the course to ensure complete drug clearance.
Long-Term Use Considerations
Extended use is rarely warranted. Chronic therapy increases the likelihood of adverse reactions including neuropathy and hematologic disturbances. Periodic monitoring is advisable if therapy must be continued beyond standard short-course regimens.
Precautions in Hepatic or Renal Impairment
Tinidazole undergoes extensive hepatic metabolism and renal excretion. Patients with compromised liver or kidney function may exhibit altered drug clearance. Dose modification and careful clinical monitoring are essential in such populations to avoid toxicity.
Careful Administration and Special Considerations
Administration in Elderly Patients
Elderly patients often present with altered pharmacokinetics due to reduced hepatic blood flow, renal function decline, and comorbidities. This can result in elevated plasma concentrations and exaggerated effects.
- Close monitoring for adverse reactions is recommended.
- Dosage adjustments may be required depending on organ function tests.
Administration in Pregnant Women
Tinidazole is contraindicated in the first trimester due to concerns about teratogenicity. In later stages of pregnancy, it may be considered when therapeutic benefits clearly outweigh potential risks. Data from animal studies reveal possible reproductive toxicity, though clinical experience in humans suggests cautious use when necessary.
Administration in Nursing Mothers
Tinidazole is excreted into breast milk in measurable amounts, posing a risk of exposure to infants. To minimize risk:
- Breastfeeding should be delayed for 72 hours after completing therapy.
- Infants should be observed for gastrointestinal disturbances or signs of toxicity if breastfeeding resumes earlier.
Administration in Children
Tinidazole is approved for pediatric use in certain parasitic infections. Dosing is carefully calculated based on body weight, typically capped at 2 g daily. Safety in infants under three months has not been conclusively established.
Long-term administration in children is discouraged, as cumulative exposure could increase risks of neurological or hematological complications.
Overdosage of Tiniba
Symptoms of Overdose
Excessive intake may produce a constellation of symptoms including severe nausea, persistent vomiting, dizziness, seizures, and peripheral neuropathy.
Emergency Management and Supportive Treatment
There is no specific antidote. Management involves stabilization of vital signs, seizure control, and correction of electrolyte imbalances. Neurological monitoring is crucial during recovery.
Role of Gastric Lavage and Activated Charcoal
If ingestion is recent, gastric lavage may be considered. Administration of activated charcoal helps limit systemic absorption, especially when performed promptly.
Storage and Handling Precautions
Recommended Storage Temperature and Conditions
Tablets and oral suspensions should be stored at controlled room temperature, generally between 20°C and 25°C. Extreme temperatures can compromise drug stability.
Protection from Light and Moisture
Store in a tightly closed container, away from direct light and humidity, as exposure can degrade the active compound and reduce efficacy.
Shelf Life and Stability Information
The typical shelf life ranges from 24 to 36 months. Suspensions, once reconstituted, may have a shorter stability window and should be used within the specified timeframe provided by the manufacturer.
Safe Handling Precautions for Healthcare Professionals
Healthcare staff should handle tinidazole with standard protective measures, avoiding inhalation of powder or contact with broken tablets. Proper disposal of unused or expired medication must follow local pharmaceutical waste regulations.
Tiniba, Tinidazole FAQ
- What is tiniba used for?
- What are the side effects of tiniba 300?
- Can tiniba be used for loose motion?
- What is tiniba 800 mg used for?
- What is tinidazole used to treat?
- Is tinidazole used for diarrhea?
- Is tinidazole a flagyl?
- Can tinidazole treat gonorrhea?
- How long does it take tinidazole to clear an infection?
- What should I avoid while taking tinidazole?
- How many tinidazole 500mg should I take?
- Is tinidazole a probiotic?
- Can I take tinidazole on an empty stomach?
- Is tinidazole the same as amoxicillin?
- Which disease is cured by tinidazole?
- What is the success rate of tinidazole?
- Can I take 4 tablets of tinidazole at once?
- Is tinidazole safe for kidneys?
What is tiniba used for?
The Tiniba 500 Tablet is a type of medication that's effective in treating various infections caused by bacteria in different parts of the body, such as the liver and stomach as well as in other organs like the brain and heart, by preventing the growth of the bacteria responsible for the infection
What are the side effects of tiniba 300?
- nausea
- vomiting
- diarrhoea
- skin rash
Can tiniba be used for loose motion?
Yes
What is tiniba 800 mg used for?
The Tiniba 800 mg Infusion is effective in reducing the risk of infection following surgery. It is also prescribed for treating diseases, as well as leg ulcers and pressure sores.
What is tinidazole used to treat?
- Trichomoniasis
- Giardiasis
- Amebiasis
Is tinidazole used for diarrhea?
Yes
Is tinidazole a flagyl?
No
Can tinidazole treat gonorrhea?
Yes
How long does it take tinidazole to clear an infection?
2-7 days
What should I avoid while taking tinidazole?
Avoid drinking alcohol
How many tinidazole 500mg should I take?
1 per day
Is tinidazole a probiotic?
No
Can I take tinidazole on an empty stomach?
No
Is tinidazole the same as amoxicillin?
Amoxycillin works by stopping bacteria from building their layer necessary for survival, and Tinidazole kills not just bacteria but also damages the DNA of other microorganisms.
Which disease is cured by tinidazole?
Bacterial vaginosis and trichomoniasis
What is the success rate of tinidazole?
92%–100%
Can I take 4 tablets of tinidazole at once?
Yes
Is tinidazole safe for kidneys?
Yes