Transamine
- Introduction to Transamine (Tranexamic Acid)
- Transamine Uses
- Off-Label Uses of Transamine
- Transamine Mechanism of Action
- Dosage and Administration Guidelines
- Composition and Available Formulations
- Common and Serious Side Effects
- Transamine Contraindications
- Warnings, Precautions, and Monitoring
- Special Considerations in Specific Populations
- Overdose and Emergency Management
- Handling and Storage Precautions
Introduction to Transamine (Tranexamic Acid)
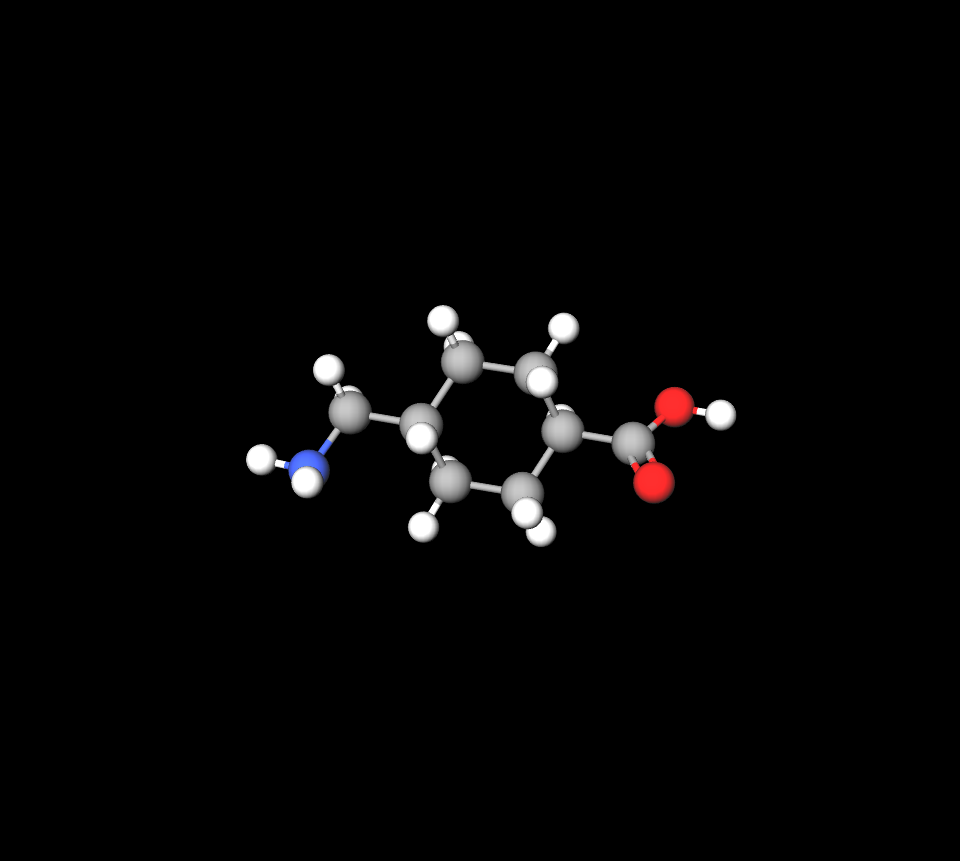
Transamine, known by its generic name tranexamic acid, is classified as an antifibrinolytic agent. It works by inhibiting the breakdown of blood clots, making it a crucial therapeutic agent in managing excessive bleeding in various clinical contexts.
Historically, tranexamic acid was synthesized in the 1960s in Japan and has since earned a well-established role in surgical, gynecological, and trauma-related bleeding control. Its clinical importance has expanded due to robust evidence supporting its safety and efficacy.
Approved by regulatory bodies including the FDA, EMA, and health authorities across Asia and the Middle East, Transamine is globally available in multiple formulations for oral, intravenous, and topical use.
The primary benefit of Transamine lies in its ability to stabilize blood clots and reduce hemorrhagic events, leading to improved outcomes in both acute and chronic bleeding conditions.
Transamine Uses
2.1 Approved Indications
- Menorrhagia: Transamine significantly reduces menstrual blood loss in women suffering from heavy periods without affecting hormonal balance.
- Hemophilia: Used as adjunct therapy in minor surgical procedures or bleeding episodes in patients with hemophilia to reduce the need for factor replacement.
- Surgical bleeding control: Frequently administered in cardiac, orthopedic, and dental surgeries to limit intraoperative and postoperative hemorrhage.
- Trauma: Plays a vital role in trauma protocols to prevent life-threatening bleeding in emergency and battlefield settings.
2.2 Use in Medical Conditions
- Postpartum hemorrhage: A life-saving option when administered within three hours after childbirth-related bleeding.
- Gastrointestinal bleeding: Utilized to reduce rebleeding rates in peptic ulcer and variceal hemorrhages.
- Epistaxis: Nasal bleeding episodes, particularly recurrent ones, may be controlled using topical or oral tranexamic acid.
- Hematuria: Reduces visible blood in urine caused by bladder or prostate conditions.
- IUD-related bleeding: Occasionally prescribed to manage breakthrough bleeding in women using intrauterine devices.
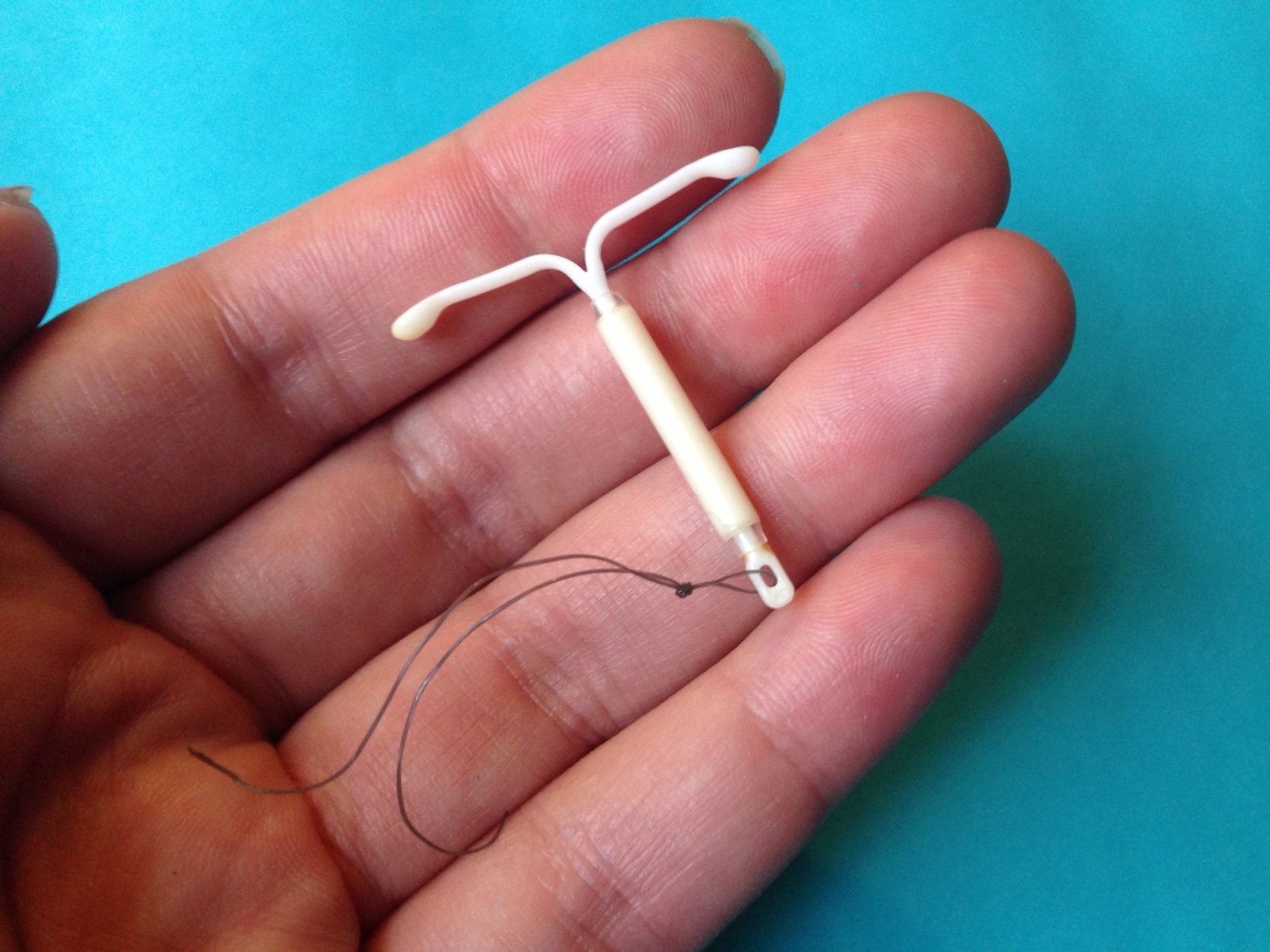
Off-Label Uses of Transamine
- Melasma and hyperpigmentation: Oral and topical forms have shown promising results in reducing skin pigmentation, likely through its anti-inflammatory effects.
- Hereditary angioedema: Serves as an adjunct to prevent recurrent swelling episodes in individuals with rare genetic disorders.
- Plastic and cosmetic surgeries: Minimizes bruising and blood loss during elective aesthetic procedures.
- Subarachnoid hemorrhage: Occasionally used in neurosurgical settings to prevent rebleeding from ruptured cerebral aneurysms.
- DIC adjunct therapy: Employed with caution in select cases of disseminated intravascular coagulation to reduce excessive fibrinolysis.
Transamine Mechanism of Action
Tranexamic acid works by reversibly binding to plasminogen, preventing its conversion into plasmi an enzyme that degrades fibrin clots. This effectively preserves the integrity of formed clots, thereby reducing bleeding.
It enhances clot stability, particularly in hyperfibrinolytic states such as trauma or surgery. In dermatology, its secondary action on melanogenesis inhibition and vascular endothelial growth factor (VEGF) suppression contributes to its efficacy in treating melasma.
Pharmacodynamically, its effects begin within 30 minutes of oral administration, with peak plasma levels reached within two hours.
Dosage and Administration Guidelines
5.1 Standard Dosage for Adults
- Oral use: Typically 500 mg to 1000 mg three times daily during menstruation for up to 5 days.
- Intravenous use: 10 mg/kg administered slowly before or during surgery, repeatable every 6-8 hours depending on bleeding severity.

5.2 Pediatric Dosage Considerations
- Doses adjusted by body weight, generally 10-15 mg/kg orally or IV every 8 hours.
- Pediatric-specific formulations or compounded liquid versions may be used where available.
5.3 Administration Timing and Duration
- Preoperative administration is often initiated 1-2 hours before incision.
- Postoperative continuation depends on bleeding risk, commonly maintained for 2-3 days.
Composition and Available Formulations
- Active ingredient: Tranexamic acid in concentrations ranging from 250 mg to 1000 mg per dose.
- Available forms: Film-coated tablets, injectable vials, capsules, oral solutions, and topical creams.
- Excipients: May include magnesium stearate, microcrystalline cellulose, or propylene glycol (topical forms).
- Brands: Sold under various names such as Transamine, Cyklokapron, Lysteda, and generics worldwide.
Common and Serious Side Effects
7.1 Common Side Effects of Transamine
- Nausea and vomiting, especially at higher doses
- Gastrointestinal discomfort and bloating
- Headaches or mild dizziness
- Back pain or muscle stiffness

7.2 Rare and Serious Adverse Effects
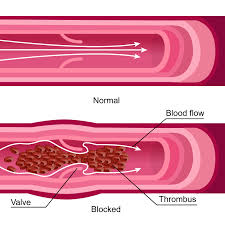
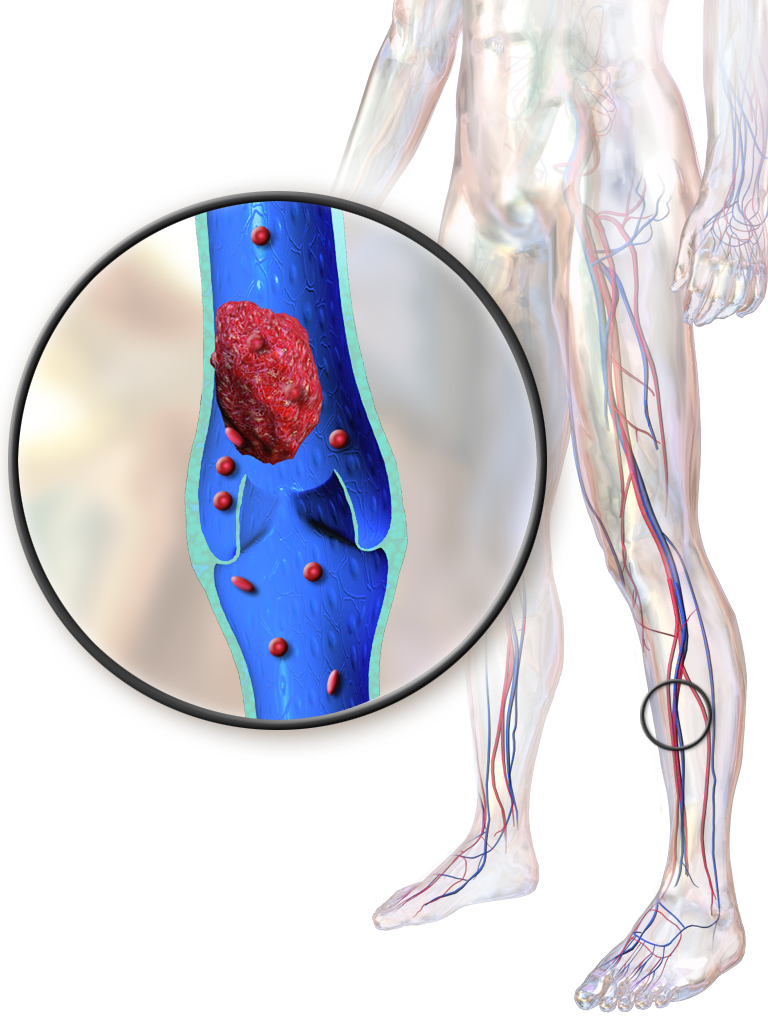
- Thrombosis: Deep vein thrombosis, myocardial infarction, or pulmonary embolism
- Ocular disturbances: Visual impairment, color vision changes
- Neurological effects: Seizures, especially following high IV doses or in renal impairment
- Allergic reactions: Rash, itching, or life-threatening anaphylaxis
Transamine Contraindications
8.1 Known Drug Interactions
- Hormonal contraceptives: May potentiate the risk of thromboembolic events.
- Anticoagulants: Interferes with the balance of hemostasis; close monitoring required.
- Thrombolytics: Direct antagonistic effects with fibrinolytic medications like alteplase.
8.2 Absolute and Relative Contraindications
- Active or historical thromboembolic disease
- Current intravascular clotting disorders or DIC without appropriate control
- Documented hypersensitivity to tranexamic acid or any formulation component
Warnings, Precautions, and Monitoring
9.1 Important Warnings
Transamine (tranexamic acid) is associated with specific clinical warnings that necessitate careful pre-treatment evaluation and ongoing patient monitoring.
- Risk of thrombosis: Due to its antifibrinolytic properties, there is an inherent risk of venous or arterial thromboembolic events. Patients with a history of deep vein thrombosis, pulmonary embolism, or ischemic heart disease should undergo a comprehensive coagulation risk assessment prior to initiation.
- Neurological concerns: High-dose or rapid intravenous administration may lower the seizure threshold, particularly in patients with renal dysfunction or a predisposing neurological condition.
- Ophthalmic complications: Long-term use requires regular vision checks, as rare instances of visual disturbances, including color vision anomalies and retinal effects, have been documented.

9.2 Precautions Before Initiating Therapy
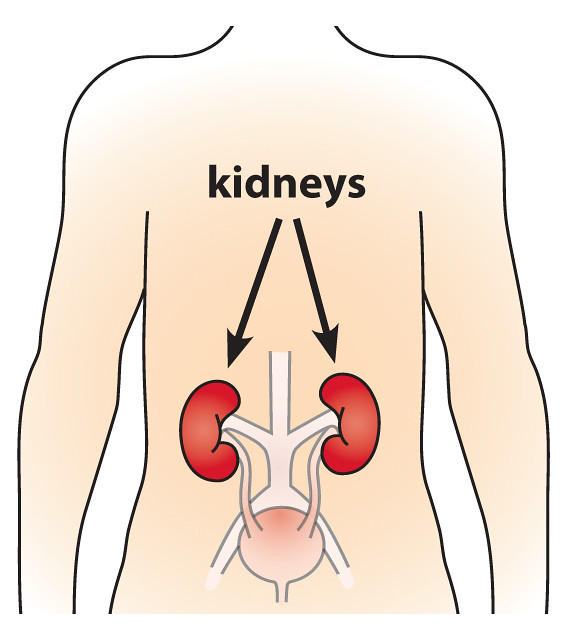
- Renal function testing: Since tranexamic acid is primarily excreted unchanged by the kidneys, baseline serum creatinine and eGFR levels should be established to guide dosing.
- Clotting history evaluation: Patients with a personal or family history of thrombophilia or clotting disorders must be evaluated with appropriate laboratory panels, including PT, aPTT, and D-dimer levels.
- Concomitant estrogen therapy: Use of estrogen-containing medications, such as oral contraceptives or hormone replacement therapy, significantly amplifies the risk of thrombotic events when used alongside tranexamic acid.
Special Considerations in Specific Populations
10.1 Administration in the Elderly
Elderly patients often present with age-related physiological changes that affect drug metabolism and excretion.
- Renal monitoring: Since renal clearance diminishes with age, frequent monitoring of kidney function is advised to prevent drug accumulation and toxicity.
- Dose adjustments: Reduced or less frequent dosing may be necessary to mitigate heightened sensitivity to side effects, such as dizziness or thrombotic complications.
10.2 Use in Pregnant and Nursing Women
Tranexamic acid is classified as pregnancy category B by the FDA, indicating no evidence of risk in animal studies, though human data remain limited.
- Placental transfer: The drug crosses the placenta, but studies have shown minimal risk to fetal development when used during the second and third trimesters for indications like postpartum hemorrhage prevention.
- Breastfeeding considerations: Tranexamic acid is excreted in low concentrations in breast milk. While considered generally safe, caution is recommended in neonates with immature renal function.
10.3 Use in Children
- Safety and efficacy: The use of Transamine in pediatric populations is supported for select indications, such as congenital bleeding disorders or during surgical procedures with high bleeding risk.
- Off-label applications: Pediatric use beyond approved indications should be guided by specialist consultation, particularly in neurosurgery or dental surgery for hemophilic patients.
Overdose and Emergency Management
Although rare, an overdose of tranexamic acid can lead to serious complications that require prompt medical attention.
- Symptoms: Common signs of overdose include nausea, vomiting, hypotension, dizziness, and in severe cases, seizures or visual disturbances.
- Clinical management: Supportive treatment is the mainstay. Gastric lavage and activated charcoal may be used in early presentation. Intravenous fluids and seizure management protocols should be implemented if necessary.
- Dialysis considerations: Although not typically required, tranexamic acid is dialyzable, and hemodialysis may be considered in patients with renal failure or severe toxicity.
- Prognosis: With timely intervention, full recovery is likely. Long-term sequelae are rare when management is appropriate and immediate.
Handling and Storage Precautions
- Storage conditions: Store tablets and injectables at room temperature (15-25°C), protected from excessive moisture and direct light. Avoid freezing injectable formulations.
- Shelf-life: Regularly check expiration dates. Stability may decrease once bottles or vials are opened, especially in multi-dose containers.
- Safe handling: Healthcare professionals should use gloves when preparing or administering intravenous solutions to minimize contact exposure. Follow aseptic techniques during preparation.
- Disposal: Unused or expired Transamine should be disposed of via pharmaceutical take-back programs or in accordance with local hazardous waste guidelines. Do not flush into wastewater systems.
Transamine FAQ
- What is the drug transamine used for?
- What does transamine do in melasma?
- Is transamin the same as tranexamic acid?
- Does Transamin stop spotting?
- How many days should you take transamine?
- What to avoid when taking tranexamic acid?
- How does tranexamic acid affect the kidneys?
- Can tranexamic acid whiten skin?
- What happens when you stop taking tranexamic acid for melasma?
- How long to take tranexamic acid for melasma?
- Can transamine stop periods?
- What is a natural alternative to tranexamic acid?
- What happens to your body when you take tranexamic acid?
What is the drug transamine used for?
It is commonly utilized to address the following symptoms: bleeding tendencies linked to hypercoagulation issues; abnormal bleeding associated with hypercoagulation; skin redness/swelling/inflammation often seen in eczema/allergic reactions/drug rashes/toxicoderma; throat pain/redness/congestion/swelling typically observed in cases of tonsillitis.
What does transamine do in melasma?
Tranexamic acid works in treating melasma involves shrinking the blood vessels in the skin and decreasing the production of melanin by changing how keratinocytes and melanocytes interact and lowering tyrosinase activity.
Is transamin the same as tranexamic acid?
Yes
Does Transamin stop spotting?
No
How many days should you take transamine?
For periods lasting four days or to address bleeding that persists beyond a week.
What to avoid when taking tranexamic acid?
Avoid using this medicine if you are currently taking it in combination with birth control, as it could heighten the risk of developing a blood clot or experiencing a heart attack or stroke.
How does tranexamic acid affect the kidneys?
Tranxemic acid's antifibrinolytic qualities could potentially damage the kidneys by promoting the development of blood clots that impact kidney vessels and lead to necrosis.
Can tranexamic acid whiten skin?
Yes
What happens when you stop taking tranexamic acid for melasma?
Melasma may return
How long to take tranexamic acid for melasma?
6 months
Can transamine stop periods?
Tranexamic acid is typically utilized to reduce the volume of blood lost during your cycle; however, it does not halt bleeding altogether.
What is a natural alternative to tranexamic acid?
Ginger capsules and myrtle fruit syrup
What happens to your body when you take tranexamic acid?
It helps to decrease bleeding by aiding in the clotting of blood.