Introduction to Wartosin Wart Remover
Overview of Wartosin: What it is and how it is used
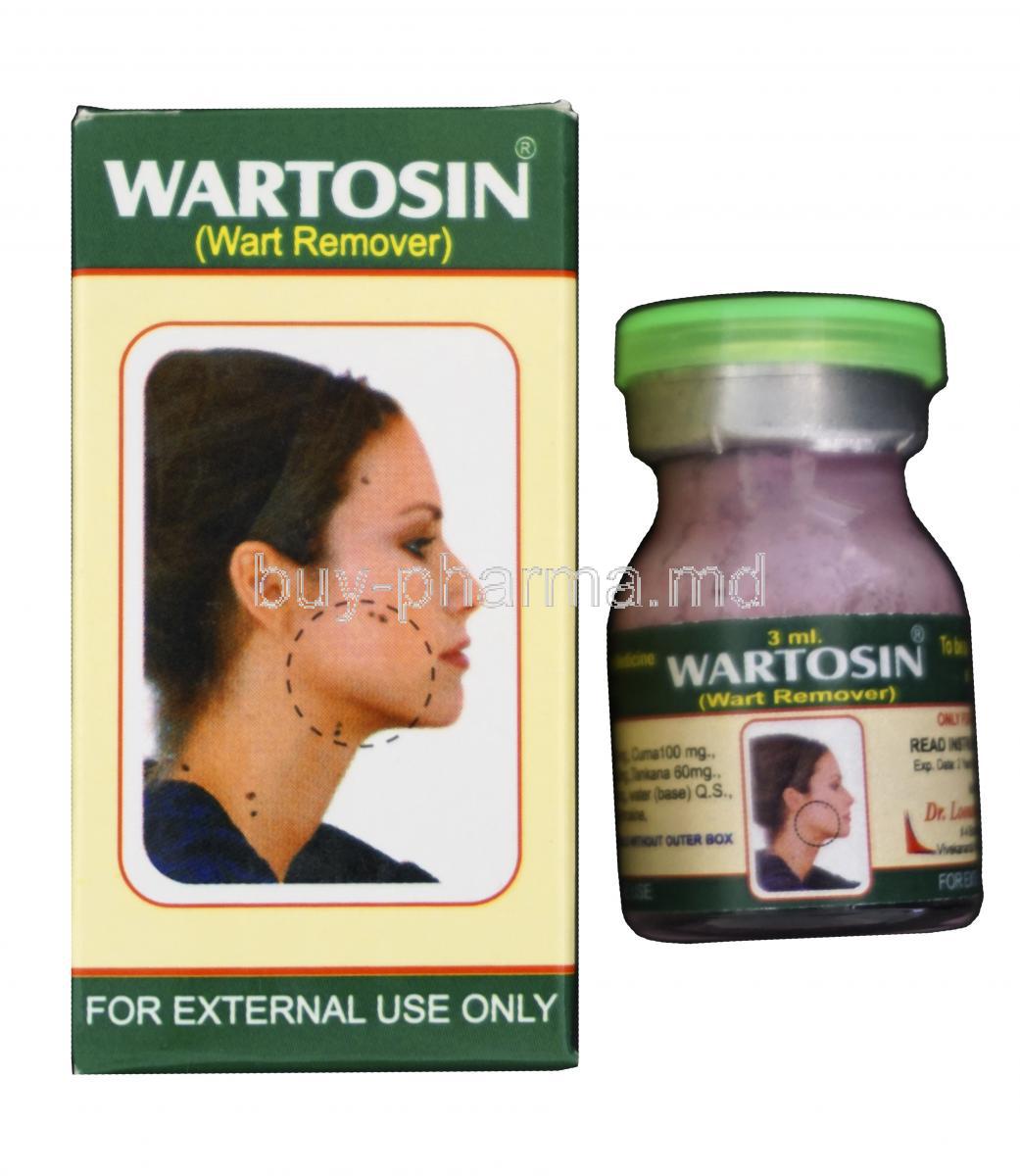
Wartosin is a topical herbal-mineral formulation designed specifically for the external treatment of warts. Applied directly to the affected skin, it works by inducing the gradual sloughing off of wart tissue. Its easy-to-use dropper bottle format allows for precise administration, making it ideal for at-home wart removal under proper guidance.
Historical background and traditional roots of Wartosin
Rooted in Ayurvedic and Siddha medical traditions, Wartosin harnesses age-old botanical remedies combined with mineral salts to treat stubborn skin growths. The formulation has evolved through generations, transitioning from traditional paste applications to a refined, standardized liquid solution for modern therapeutic use.
Regulatory status and availability (OTC vs prescription)
Wartosin is generally available as an over-the-counter (OTC) product in countries where herbal medicines are regulated under traditional systems. However, in some jurisdictions, its use may be guided by practitioner advice due to the caustic nature of the formulation. Always check local regulations before purchase or use.
Medical and Off-Label Uses of Wartosin Wart Remover
Primary indication: Removal of cutaneous warts (common, plantar, flat, filiform)
Wartosin is primarily indicated for the removal of various types of cutaneous warts including:
- Common warts (verruca vulgaris)
- Plantar warts on the soles of the feet
- Flat warts, particularly on the face or hands
- Filiform warts, often found around the mouth or eyes
Use for genital warts: With caution and under medical supervision
While not formally approved for genital wart treatment, Wartosin has been used off-label under strict dermatological supervision. Due to the sensitive nature of mucosal tissue, application in these areas is generally discouraged without medical oversight.
Off-label use for skin tags and corns
Some practitioners and users apply Wartosin to skin tags and corns, citing anecdotal evidence of efficacy. However, these uses fall outside standard recommendations and carry a risk of irritation or chemical burn.
Use in cosmetic dermatology for benign keratotic lesions
Beyond wart removal, Wartosin may be employed by traditional practitioners to address benign keratotic lesions as part of cosmetic regimens, particularly when surgical removal is undesirable. Caution is advised in such use cases.
How Wartosin Wart Remover Works: Mechanism of Action
Caustic and keratolytic action on wart tissue
Wartosin exerts a caustic effect on the stratum corneum, softening and breaking down the thickened keratinized tissue of the wart. This keratolytic property facilitates gradual detachment of the lesion.
Local necrosis of wart cells and subsequent desquamation
By inducing localized necrosis at the application site, the formulation targets wart cells directly. This leads to desquamation—the shedding of dead skin—revealing healthy tissue beneath.
Role of herbal and mineral compounds in targeted action
The synergistic blend of herbal acids and mineral salts acts at the cellular level to disrupt wart proliferation. These ingredients collectively dehydrate, shrink, and ultimately destroy wart tissue without systemic effects.
Duration until visible wart removal
Treatment response typically begins within 3–5 days, with complete wart detachment occurring in 7–15 days depending on size and location. Reapplication may be needed for stubborn lesions.
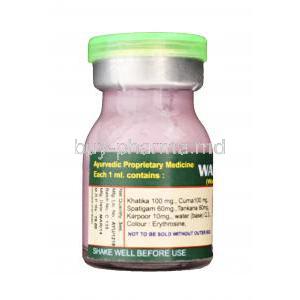
Composition and Active Ingredients in Wartosin
Breakdown of herbal and mineral constituents (e.g., copper sulfate, herbal acids)
Key ingredients include:
- Copper sulfate: Induces coagulative necrosis of wart tissue
- Herbal acids: Provide keratolytic and astringent properties
- Plant-based alkaloids: Disrupt abnormal cellular activity
Description of excipients and formulation base
The solution is typically suspended in a water-alcohol base, ensuring rapid absorption and improved shelf stability. Emulsifiers and stabilizers may be used to enhance consistency and prevent crystallization.
Role of each ingredient in wart removal
Each component is selected for its unique pharmacodynamic effect:
- Minerals destroy infected keratinocytes
- Herbal acids penetrate deep into the wart
- Emollients protect surrounding skin from damage
Comparison with other wart treatments
Unlike salicylic acid or cryotherapy, Wartosin uses a plant-mineral synergy to gradually eliminate warts. While slower-acting, it may be preferred for patients seeking natural alternatives or who are intolerant to freezing agents.
Dosage and Administration Guidelines for Wartosin
Step-by-step application procedure
- Clean the affected area with warm water and soap.
- Dry the area thoroughly.
- Apply a thin layer of Wartosin using a cotton swab or applicator.
- Avoid surrounding healthy skin.
- Let it dry; do not cover with a bandage.
Recommended frequency and duration of use
Apply once daily for 5–7 consecutive days. For larger or thicker warts, treatment may extend up to 2 weeks. If no improvement is seen, a second course may be considered after consultation.
Special application instructions for plantar and facial warts
Due to the thickness of plantar skin, gentle abrasion before application may enhance penetration. For facial warts, extreme caution is advised to prevent irritation or scarring. Avoid areas near the eyes.
Guidelines for repeated treatment cycles
If complete clearance is not achieved after the first cycle, a gap of 7–10 days is recommended before repeating treatment. Do not apply more than two consecutive cycles without medical supervision.
Storage Instructions and Shelf Life of Wartosin
Ideal storage temperature and conditions
Store in a cool, dry place between 15°C and 25°C. Keep the container tightly closed and away from direct sunlight to preserve the chemical integrity of the active ingredients.
Container handling and sealing precautions
Ensure the cap is replaced immediately after use. Avoid touching the nozzle or dropper tip to prevent contamination. Always keep the bottle upright.
Shelf life and signs of product degradation
Shelf life is typically 24 months from the date of manufacture. Discard if the solution changes color, precipitates, or develops an unusual odor.
Common and Serious Side Effects of Wartosin
Common side effects: redness, stinging, skin irritation
Mild localized symptoms such as burning, tingling, and transient redness may occur. These are typically self-limiting and resolve upon discontinuation.
Serious adverse effects: ulceration, chemical burns, scarring
Over-application or misuse can cause deeper skin injury including ulceration and scarring. Immediate cessation is advised if such effects develop.
Allergic reactions and symptoms to watch for
Allergic contact dermatitis, swelling, and severe itching may indicate hypersensitivity. Stop use and consult a physician if these symptoms arise.
Frequency and risk factors for side effects
Adverse effects are more likely in individuals with sensitive skin, prolonged exposure, or misuse. Using barrier creams can minimize risk.
Drug and Product Interactions with Wartosin
Interactions with other topical medications (e.g., retinoids, corticosteroids)
Avoid concurrent use with strong topical agents, which may exacerbate irritation or reduce efficacy. Space applications by several hours if necessary.
Impact of simultaneous use with emollients or occlusive agents
Occlusive dressings may intensify absorption and should generally be avoided. Emollients may dilute the product or alter its action.
Potential interactions with systemic immunosuppressants
Though topical, caution is advised in patients on immunosuppressive therapy, as local immune response may be altered, affecting efficacy.
Contraindicated cosmetic procedures during treatment
Avoid chemical peels, microdermabrasion, or laser therapy on the treated area during the course of Wartosin application.
Warnings and Contraindications for Wartosin Use
Contraindications: Open wounds, inflamed skin, known hypersensitivity
Do not use on broken, bleeding, or actively inflamed skin. Contraindicated in those with known allergies to any of the product’s ingredients.
Warnings regarding mucosal application and eye contact
Strictly avoid contact with eyes, mucous membranes, or internal surfaces. If accidental exposure occurs, rinse thoroughly and seek medical advice.
Caution in use for large or clustered wart areas
Treat only a few warts at a time to prevent systemic absorption or localized toxicity. Break treatments into smaller sections if needed.
Not recommended for immunocompromised individuals without medical advice
Patients with compromised immunity may experience delayed healing or increased risk of secondary infection. Professional supervision is recommended.
Precautions and Careful Administration Considerations
Skin patch test recommendation before full application
Apply a small amount to unaffected skin on the forearm and monitor for 24 hours. Proceed only if no reaction occurs.
Use of protective barrier creams around the application site
Apply petroleum jelly or a zinc-based cream around the wart to shield surrounding skin from potential irritation or damage.
Avoidance of over-application or prolonged exposure
Excess product does not accelerate healing and may lead to adverse effects. Adhere strictly to recommended dosages and duration.
Safety tips for self-application at home
Wash hands thoroughly before and after use. Keep out of reach of children. Use in a well-ventilated area and store securely after each use.
Use in Special Populations
Administration to Elderly Patients
Elderly skin tends to be thinner and more vulnerable. Lower concentrations or reduced frequency of application may be required.
Recommendation for dermatologist supervision
Due to age-related dermal sensitivity, a dermatologist should ideally monitor application in geriatric patients to minimize complications.
Use in Pregnant Women and Nursing Mothers
The safety profile of Wartosin during pregnancy or lactation has not been established. Avoid use unless the potential benefit outweighs the risk.
Potential risks to fetus and nursing infant
Although systemic absorption is minimal, inadvertent ingestion or dermal exposure in infants could pose unknown risks. Medical consultation is mandatory.
Consultation with healthcare provider required
Pregnant or breastfeeding women should not initiate use without a formal risk-benefit assessment by a qualified physician.
Use in Pediatric Patients
Wartosin is not typically recommended for children under 12 unless specifically prescribed. Their skin is more permeable and reactive.
Recommended usage only under medical supervision
If treatment in children is necessary, ensure it is done under pediatric dermatology supervision with adjusted application frequency.
Overdose and Accidental Misuse of Wartosin
Signs of overdose or excessive application
Look for severe skin peeling, blistering, or ulceration. Increased pain or bleeding at the site may also occur.
Immediate first aid measures and rinsing protocols
Flush affected area with cold water for several minutes. Apply soothing emollients and discontinue use immediately.
When to seek emergency medical care
Persistent burning, infection, or systemic symptoms like dizziness or shortness of breath necessitate urgent medical evaluation.
Long-term consequences of misuse
Improper use can lead to permanent scarring, pigmentation changes, or chronic skin sensitivity. Always adhere to labeled instructions.
Handling Precautions and Safe Disposal
Use of gloves and applicators to avoid direct contact
To prevent unintentional exposure, wear disposable gloves or use cotton-tipped applicators. Avoid skin-to-product contact.
Cleaning of skin and hands after application
Immediately rinse hands with soap and water after application. Clean the surrounding skin gently to remove excess product.
Proper disposal of leftover product and containers
Do not flush unused product. Dispose in accordance with local hazardous waste guidelines. Seal bottles tightly before discarding.
Environmental safety considerations
Avoid disposal near water sources. The product may pose risks to aquatic organisms if released into the environment in large amounts.