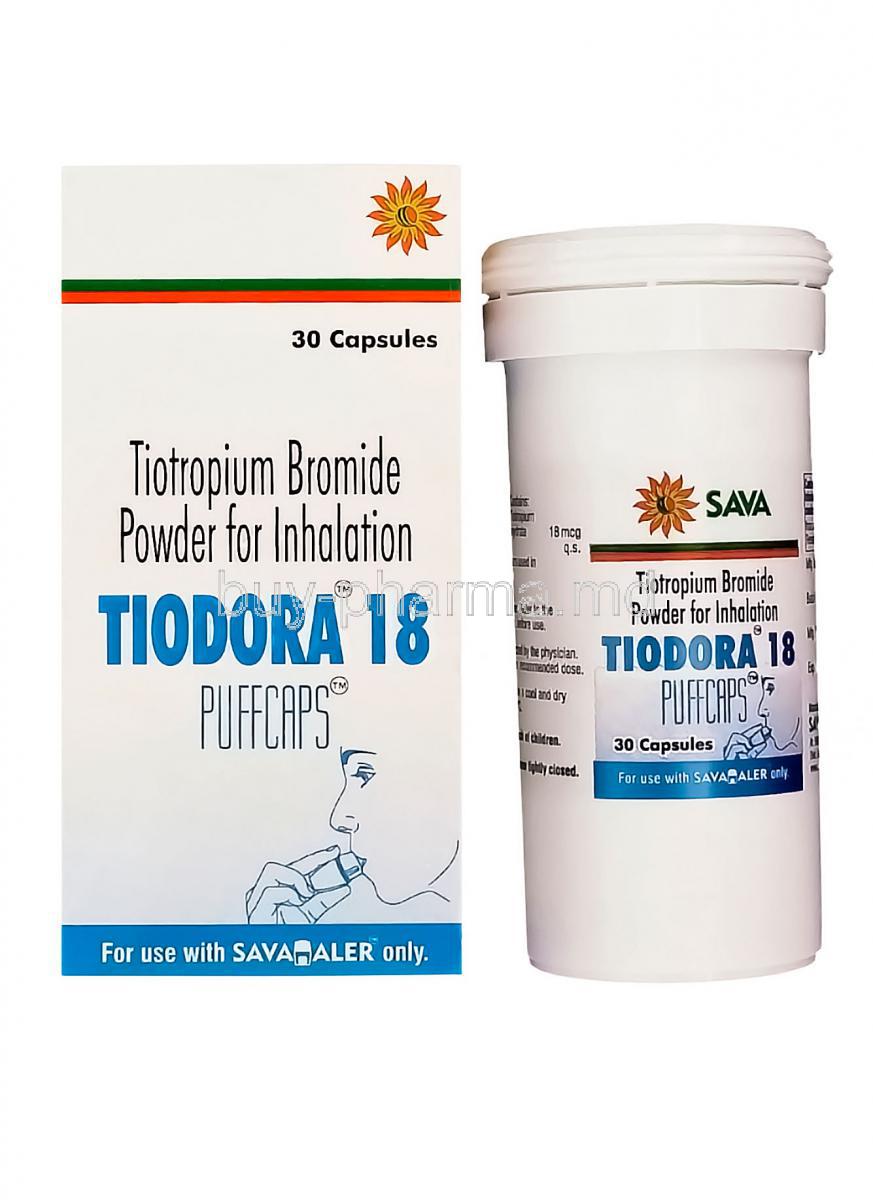
Introduction to Tiodora Pufcaps
Tiodora Pufcaps is a specialized formulation of Tiotropium Bromide, designed to deliver targeted relief in chronic respiratory disorders. Classified under the therapeutic category of long-acting muscarinic antagonists (LAMA), it plays a vital role in maintaining clear airways for individuals suffering from persistent breathing difficulties.
The medication is particularly significant in the management of chronic conditions such as COPD and asthma, where airflow limitation is a primary concern. By offering long-lasting bronchodilation, it helps patients maintain better control of their symptoms, reduces flare-ups, and improves overall quality of life. The inhalation route of administration provides direct action in the lungs, ensuring faster onset and fewer systemic side effects compared to oral therapy.
Composition and Formulation
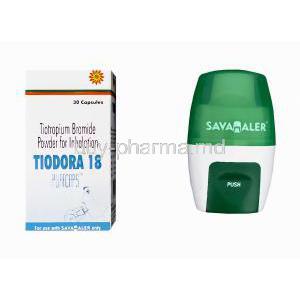
The principal active component in Tiodora Pufcaps is Tiotropium Bromide, a highly potent muscarinic receptor antagonist. The Pufcaps formulation employs a dry powder inhalation system, delivering precise doses directly to the lungs where the therapeutic impact is most effective.
- Active ingredient: Tiotropium Bromide
- Delivery system: Pufcaps dry powder inhalation
- Inactive excipients: stabilizers and carriers that ensure consistent absorption
- Available dosage strengths to accommodate varied patient needs
Mechanism of Action: How Tiodora Pufcaps Works
Tiodora Pufcaps acts by blocking muscarinic receptors in the airway smooth muscle. This antagonism prevents acetylcholine-mediated bronchoconstriction, leading to relaxation of bronchial muscles and a sustained opening of the airways.
- Promotes effective bronchodilation and enhances airflow
- Reduces bronchospasm and limits constrictive episodes
- Provides 24-hour efficacy, making once-daily dosing sufficient
The prolonged duration of action ensures consistent control of respiratory symptoms, reducing the dependency on rescue medications.
Approved Medical Uses of Tiodora Pufcaps
Chronic Obstructive Pulmonary Disease (COPD)
Tiodora Pufcaps is widely prescribed for the long-term maintenance treatment of COPD, including chronic bronchitis and emphysema.
- Improves lung function and relieves persistent dyspnea
- Decreases the frequency and severity of exacerbations
- Supports sustained respiratory stability in daily living
Asthma Management
In severe persistent asthma, Tiodora Pufcaps may be used as an add-on therapy when standard treatments are insufficient.
- Improves lung performance in difficult-to-control asthma
- Enhances quality of life by reducing nocturnal symptoms
- Offers additional bronchodilation alongside inhaled corticosteroids
Off-Label Uses of Tiotropium Bromide
While not officially approved for all respiratory conditions, ongoing research highlights potential off-label applications:
- Adjunctive therapy in cystic fibrosis for improved airflow
- Relief in bronchiectasis to reduce sputum accumulation
- Symptom management in chronic bronchitis not classified under COPD
- Investigational role in certain forms of allergic asthma
Dosage and Administration Guidelines
Standard Adult Dosage
The usual recommended dose is a once-daily inhalation using the Pufcaps device. Proper inhalation technique is critical to ensure maximum therapeutic effect.
Dosage Adjustments in Specific Populations
- Renal impairment: dose adjustments may be necessary due to altered excretion
- Hepatic impairment: caution advised, though metabolism is limited
- Elderly patients: no major changes, but monitoring is recommended
Missed Dose and Overuse Instructions
- If a dose is missed, take it as soon as remembered unless it is close to the next scheduled dose
- Avoid double dosing as it increases the risk of adverse effects
Side Effects of Tiodora Pufcaps
Common Side Effects
- Dry mouth and throat irritation
- Cough, hoarseness, and nasal dryness
- Mild headache and dizziness
Serious or Rare Side Effects
- Paradoxical bronchospasm, requiring immediate medical attention
- Urinary retention, particularly in patients with prostate enlargement
- Cardiovascular events such as palpitations or arrhythmias
- Severe hypersensitivity reactions including rash and angioedema
Drug Interactions with Tiodora Pufcaps
- Should not be combined with other anticholinergic drugs due to additive effects
- Caution with concomitant use of beta-agonists and inhaled corticosteroids
- Potential interactions with diuretics and cardiovascular agents
- Food and alcohol have minimal effect, but excessive use may aggravate side effects
Warnings and Contraindications
Absolute Contraindications
Tiodora Pufcaps should never be administered to individuals with a known hypersensitivity to tiotropium bromide or atropine derivatives. Allergic manifestations may include severe rash, swelling, or respiratory distress. Additionally, patients with narrow-angle glaucoma face significant risks, as the anticholinergic properties of tiotropium may exacerbate intraocular pressure, leading to acute ocular emergencies.
Major Warnings
While generally well-tolerated, specific patient groups require heightened vigilance:
- Acute bronchospasm: Although intended to prevent airway constriction, paradoxical bronchospasm may occur in rare cases, necessitating discontinuation and urgent medical care.
- Bladder obstruction or prostatic hypertrophy: Anticholinergic effects may precipitate urinary retention, worsening pre-existing urological conditions.
- Cardiovascular disease: Caution is warranted in patients with arrhythmias, hypertension, or ischemic heart conditions, as tiotropium may influence heart rate and cardiac conduction.
Careful Administration and Precautions
Careful administration ensures optimal therapeutic benefit while minimizing risk. Monitoring parameters must be considered throughout therapy.
- Renal impairment: Since tiotropium is primarily excreted unchanged via the kidneys, accumulation may occur in renal dysfunction, necessitating dosage vigilance and clinical monitoring.
- Cardiovascular comorbidities: Patients with coexisting cardiac conditions should be observed for signs of arrhythmia or palpitations during long-term use.
- Long-term safety: Prolonged therapy requires periodic reassessment of benefit versus potential anticholinergic burden, particularly in polypharmacy scenarios.
Administration in Special Populations
Elderly Patients
The safety and efficacy of Tiodora Pufcaps are generally consistent in older adults. However, polypharmacy increases the likelihood of drug-drug interactions. Regular medication reviews help to reduce adverse outcomes and optimize therapy.
Pregnant Women and Nursing Mothers
The use of tiotropium during pregnancy requires caution. Limited data are available on placental transfer, and while animal studies have not demonstrated teratogenicity, human evidence is insufficient. During lactation, trace excretion into breast milk is possible, raising potential concerns for nursing infants.
- Prescribe only if benefits outweigh potential risks
- Prefer alternative agents in high-risk pregnancies when feasible
- Monitor neonates for signs of anticholinergic exposure if maternal therapy is continued
Pediatric and Adolescent Patients
Tiodora Pufcaps is not universally indicated for children. Age-related restrictions limit its use, with approval typically confined to severe asthma cases where other therapies have proven insufficient. Long-term pediatric safety data remain limited, and therapy should only proceed under specialist supervision.
Overdosage and Emergency Management
Excessive use of Tiodora Pufcaps may provoke significant anticholinergic symptoms:
- Dry mouth and intense thirst
- Constipation and abdominal discomfort
- Blurred vision and ocular discomfort
- Urinary retention with potential bladder distension
Immediate supportive measures include discontinuation of the drug and hydration support. In severe cases, emergency interventions such as catheterization for urinary retention or cardiovascular monitoring for arrhythmias may be necessary.
Storage and Handling Precautions
- Storage conditions: Maintain in a cool, dry place away from direct sunlight and humidity. Excessive moisture may compromise the integrity of dry powder formulations.
- Shelf life: Use before the expiration date indicated on the packaging to ensure potency and safety.
- Disposal: Expired or unused capsules should be discarded responsibly, avoiding household waste where children or pets may gain access.
- Handling: Ensure hands are dry before handling capsules. Do not swallow capsules directly; they are intended exclusively for inhalation via the Pufcaps device.