Introduction to Emeset (Ondansetron)
Overview of Emeset brand and Ondansetron as the active ingredient
Emeset is a widely utilized antiemetic brand that contains Ondansetron as its principal therapeutic component. This pharmacological agent is recognized globally for its ability to counteract complex nausea pathways. It is used in clinical settings where a targeted blockade of nausea neurotransmission is required.
Ondansetron is structurally classified as a substituted carbazole derivative, formulated to provide predictable suppression of nausea triggers. It offers an efficient intervention where conventional antinauseants fail.
Therapeutic category: 5-HT3 receptor antagonist antiemetic
Ondansetron belongs to the 5-HT3 receptor antagonist class – a niche category of antiemetics that directly impede serotonin-mediated responses in central and peripheral pathways. These agents have a long-standing reputation for efficacy in procedural or therapy-associated nausea syndromes.
Clinical relevance in modern anti-nausea and anti-emesis protocols
Contemporary oncology, surgical, and radiation protocols routinely integrate Emeset to enhance patient comfort and adherence to therapy regimens. Its use has substantially reduced interruption of chemotherapy and postoperative recovery delays. It is now considered indispensable in refined supportive care.
Composition and Available Pharmaceutical Formulations
Active ingredient concentration and chemical classification
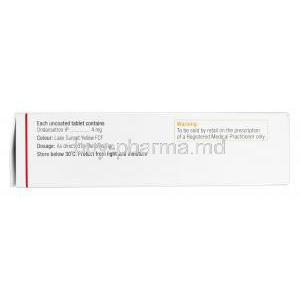
Emeset products commonly contain Ondansetron hydrochloride or Ondansetron hydrochloride dihydrate in quantifiable standardized strengths such as 4 mg and 8 mg.
Common dosage forms: tablets, oral dispersible tablets, injectables
Commercially accessible pharmaceutical formats include:
- Standard swallow tablets
- Fast dissolving oral dispersible tablets
- Intravenous and intramuscular injectable solutions
Excipients and formulation differences between generic vs branded Emeset
Inactive constituents may vary. Some variations include sweetening agents, dispersible carrier matrices, stabilizers, or isotonic buffering excipients. Branded Emeset products may demonstrate finer solubility characteristics compared to certain generics, facilitating smoother patient acceptability.
Mechanism of Action – How Ondansetron Works
Selective 5-HT3 receptor blockade in the CNS and GI tract
Ondansetron exerts a selective antagonistic influence upon peripheral vagal terminals in the gastrointestinal environment, while simultaneously acting on central chemoreceptor trigger zones. This dual locus blockade positions it as a highly strategic antiemetic.
Role in suppression of serotonin-mediated nausea signaling pathways
Serotonin is a dominant mediator of emetogenic signaling. When serotonin surges due to cytotoxic chemotherapy, radiation exposure, or surgical stimulus, 5-HT3 mediated activity escalates. Ondansetron interrupts this cascade, preventing nausea onset and diminishing vomiting impulses.
Onset of action and pharmacokinetic profile overview
Typical onset occurs within 15–30 minutes post oral ingestion. Injectable routes may yield even more rapid efficacy. Distribution is systemic and hepatic metabolism is prominent, producing active metabolites with clinically relevant persistence.
Primary Uses and Approved Medical Applications
Prevention of chemotherapy-induced nausea and vomiting (CINV)
This is the cornerstone application for Ondansetron. It reduces the emetogenic burden during cytotoxic drug cycles and contributes to measurable improvement in therapy compliance.
Control of radiation-induced nausea and vomiting (RINV)
Radiotherapy patients often experience nausea triggered by abdominal or cranial irradiation. Emeset is frequently co-prescribed to alleviate this response, improving tolerance of ongoing radiation plans.
Postoperative nausea and vomiting prevention (PONV)
After anesthesia or surgical manipulation, patients may experience abrupt nausea episodes. Emeset mitigates these postoperative disturbances and supports faster recovery trajectories.
Off-Label Use and Alternative Clinical Applications
Viral gastroenteritis-induced nausea in adults and children
In select gastroenteritis scenarios, especially those characterized by uncontrollable vomiting, Ondansetron has been judiciously employed to reduce dehydration risk and improve oral hydration capability.
Severe nausea associated with migraine attacks
Migraine-associated emesis is particularly distressing. Some clinicians integrate Ondansetron as an adjunctive measure to reduce nausea burden and improve tolerance to oral migraine abortive therapies.
Hyperemesis gravidarum use considerations (risk/benefit case-specific)
Hyperemesis gravidarum presents a unique therapeutic dilemma. Ondansetron is used on a risk-benefit basis in complex pregnancy nausea cases where malnutrition or electrolyte instability may occur. Clinical judgement is paramount and individualized dosing is emphasized.
```html
Dosage and Administration Guidelines
Standard dosing for CINV, RINV, and postoperative nausea
Standardized regimens vary according to indication, but evidence-based protocols remain consistent across surgical and oncology institutions.
- CINV adult oral dosing typically begins prior to chemotherapy commencement and is repeated at scheduled intervals.
- RINV dosing is usually initiated before radiation exposure sessions to prevent episodic nausea.
- Postoperative nausea prevention is often implemented intraoperatively or immediately after anesthesia cessation.
Timing is essential. Administration prior to the emetogenic trigger enhances efficacy markedly.
Oral vs injectable dosing routes and timing relative to trigger exposure
Oral formulations are frequently preferred for planned treatment cycles. Injectables are favored when rapid onset or impaired oral intake is expected. The route selection depends on clinical context, gastrointestinal integrity, and urgency.
Maximum daily dosage considerations and duration limits
Daily dose caps should not be exceeded. Although therapeutic windows are relatively broad, long-term unregulated usage is not recommended.
- Short-term durations aligned with procedure- or treatment-associated events are optimal.
- Continuous multi-week use requires clinical supervision due to hepatic metabolism pathways.
Administration Considerations in Special Populations
Administration to elderly patients – dosage adjustments and monitoring
In geriatric populations, physiological reserve is reduced. Lower starting doses may be advisable. Clinical teams should monitor electrolyte status and cardiac conductivity parameters for safety continuity.
Administration to pregnant women – fetal safety data and trimester considerations
Pregnancy use is evaluated case by case. Benefit must outweigh any theoretical risk. The most critical assessment periods occur in early gestation, where fetal organogenesis is underway.
Use in breastfeeding mothers – excretion into breast milk and recommendations
Small amounts of the drug may transfer into milk. Transient exposure is considered low risk in many scenarios, but breastfeeding timing adjustments may be recommended immediately post-administration to minimize neonatal exposure.
Administration to children – pediatric dosing ranges and age-specific cautions
Pediatric dosing follows weight-based calculations. Caution is emphasized in neonates and young infants due to immature metabolic enzyme capacity, making prescriber guidance mandatory.
Common Side Effects and Expected Tolerability Profile
Typical mild reactions: headache, dizziness, constipation
Mild symptoms are the most frequently observed responses. They generally dissipate without intervention and rarely require therapy cessation.
GI changes and taste alterations
Some individuals report transient dysgeusia, a metallic or unusual flavour profile, or momentary changes in bowel frequency or consistency.
Frequency and duration of typical side effect patterns
Most reactions are short-lived. They often resolve within hours as serum concentration stabilizes.
Serious Adverse Effects and Warnings
QT-interval prolongation and arrhythmia risk
A prolongation of ventricular repolarization can occur. ECG surveillance is recommended for individuals with prior electrical instability.
Serotonin syndrome potential when combined with serotonergic agents
Co-administration with SSRI or SNRI medications may elevate serotonin concentrations, potentially precipitating agitation, neuromuscular hyperactivity, and autonomic dysregulation.
Liver function disturbance and hepatic impairment cautions
Hepatic metabolism is the primary elimination route. Individuals with impaired hepatic pathways require restricted dosage or alternate therapy algorithms.
Drug Interactions and Concomitant Medication Use
Interaction risk with SSRI / SNRI antidepressants
Concurrent therapy can increase serotonin potentiation, elevating risk of serotonin toxicity.
CYP3A4 / CYP2D6 metabolic pathway modifiers
Inhibitors or inducers of these pathways may alter serum exposure. Pharmacokinetic variability should be accounted for before dose selection.
Concomitant use with QT-prolonging agents
Stacked risk factors amplify arrhythmia potential. Avoid unnecessary overlap.
Contraindications and When Not to Use Ondansetron
Known hypersensitivity to ondansetron or similar 5-HT3 antagonists
Any previous hypersensitivity is a clear exclusion criterion.
Congenital long QT syndrome
Underlying electrical disorders heighten the potential for torsadogenic activity.
Severe hepatic impairment absolute restrictions
Patients with advanced liver compromise cannot safely metabolize the drug, making its use inappropriate.
Important Precautions and Careful Administration
Caution in electrolyte imbalance risk states (hypokalemia / hypomagnesemia)
Electrolytes influence cardiac conduction. Monitoring is prudent in oncology patients or those receiving diuretics.
Avoiding misuse or prolonged unsupervised therapy
Antiemetics should not mask undiagnosed gastrointestinal pathology. Overreliance should be discouraged.
Assessing individual risk in oncology settings
Regimens differ substantially between chemotherapeutic agents. Supportive medications should be chosen selectively based on emetogenic potential.
Overdose Presentation and Emergency Management
Clinical manifestations of excessive ondansetron intake
Overdose symptoms may include severe dizziness, hypoactivity, or escalated cardiac conduction irregularities.
Cardiac and neurological symptom escalation risk
Electrical conduction disruption may intensify abruptly. Neurological confusion or agitation may also manifest.
Acute emergency management and hospital intervention protocols
Urgent medical evaluation is imperative. Cardiac monitoring, supportive therapy, and symptom-directed intervention are prioritized.
Storage and Handling Precautions
Protection from moisture, light, and heat for tablets
Store tablets in airtight conditions. Light and humidity accelerate deterioration.
Injectable handling and proper cold chain considerations where applicable
In injectable formats, temperature stability is critical to maintain potency. Facilities should follow controlled storage policy.
Safe handling and disposal guidance for households and clinics
Unused or expired medicines should be discarded responsibly. Avoid general waste disposal. Utilize designated pharmaceutical collection systems where available.