Apidra Solostar Pen-Injection
- Introduction to Apidra Solostar Pen-Injection
- Approved Medical Uses of Apidra Solostar
- Off-Label and Investigational Uses of Apidra Solostar
- Mechanism of Action: How Apidra Works in the Body
- Dosage and Administration Guidelines
- Composition and Formulation Details
- Common and Serious Side Effects
- Drug Interactions and Contraindicated Medications
- Warnings and Precautions for Safe Use
- Contraindications to Apidra Solostar Use
- Guidelines for Careful Administration
- Important Precautions Before and During Use
- Storage and Handling Requirements
- Use in Special Populations
- Overdose Risks and Emergency Management
- Handling and Disposal Precautions
Introduction to Apidra Solostar Pen-Injection
Apidra Solostar Pen-Injection delivers insulin glulisine, a rapid-acting insulin analog engineered for swift glucose regulation. Designed to closely mimic the body's natural postprandial insulin response, it offers an effective tool for glycemic control.
This pre-filled Solostar pen provides a user-friendly alternative to traditional vial and syringe methods. Its portability and ease of administration enhance treatment adherence, especially in outpatient and home-based care settings.
Compared to other rapid-acting insulins such as insulin lispro and insulin aspart, Apidra exhibits a slightly faster onset and shorter duration of action, making it particularly suitable for controlling blood sugar spikes following meals.
Approved Medical Uses of Apidra Solostar
- Type 1 Diabetes Mellitus: Used as part of a basal-bolus regimen for immediate postprandial glucose control.
- Type 2 Diabetes Mellitus: Administered in combination with long-acting insulin or oral hypoglycemics when meal-time glucose spikes persist.
- Inpatient Use: Frequently utilized in hospitals to manage hyperglycemia in acute care scenarios, including surgery and infection-induced stress hyperglycemia.
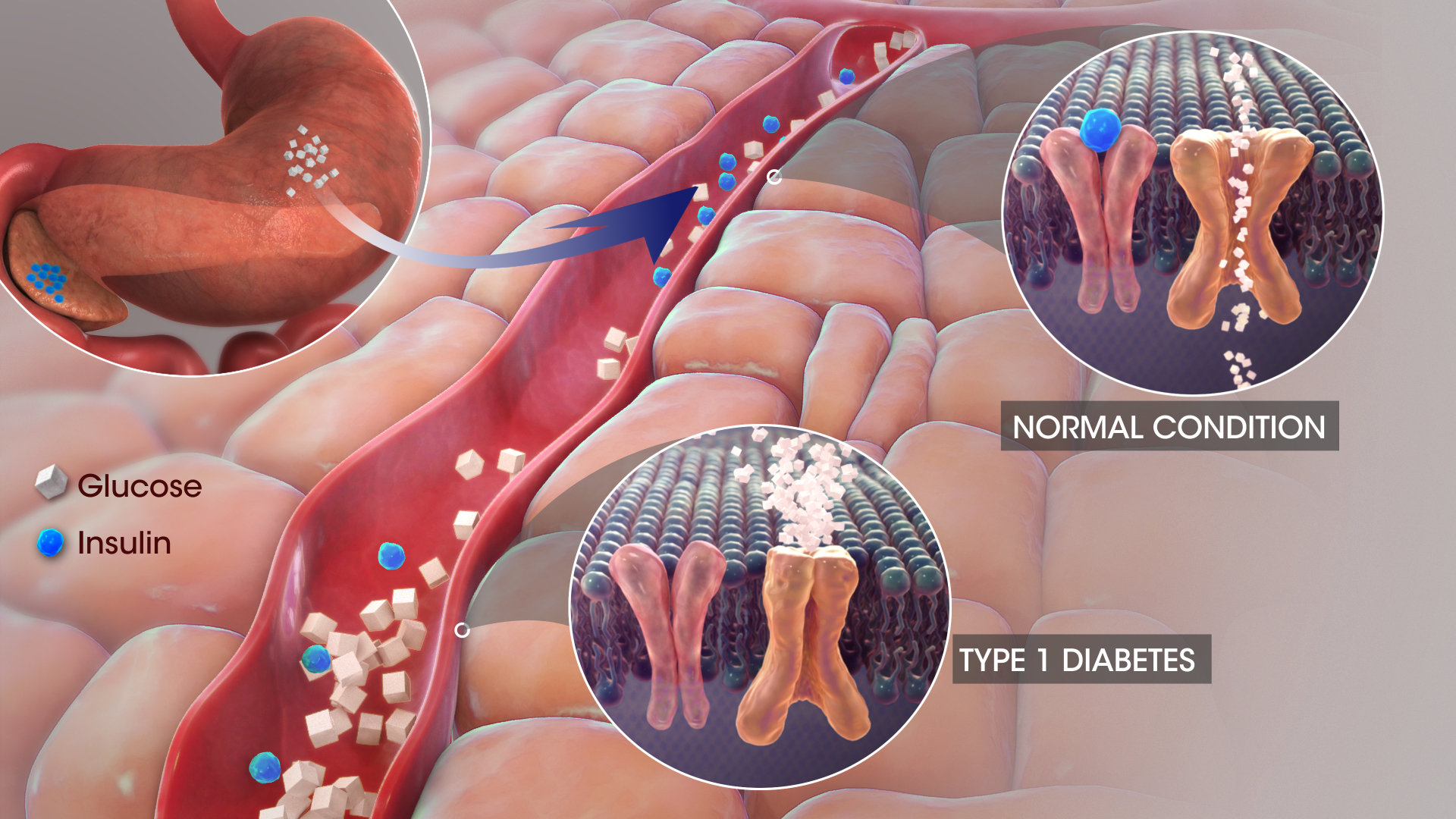
Off-Label and Investigational Uses of Apidra Solostar
- Gestational Diabetes: Applied cautiously under specialist supervision to prevent postprandial hyperglycemia.
- Children Below Approved Age: Occasionally used off-label in younger pediatric populations when no alternatives are available.
- Insulin Pump Therapy: Used in continuous subcutaneous insulin infusion (CSII) devices due to its rapid pharmacokinetics.
- Diabetic Ketoacidosis: An adjunct to IV insulin protocols during resolution phases of DKA management.

Mechanism of Action: How Apidra Works in the Body
Apidra rapidly lowers blood glucose by binding to insulin receptors, facilitating glucose uptake in skeletal muscle and adipose tissue while inhibiting hepatic glucose production. Its absorption begins within 15 minutes post-injection and peaks within 30 to 90 minutes, with a duration of action typically under 4 hours.
Unlike regular human insulin, Apidra is structurally modified at the B3 and B29 positions, enhancing solubility and speed of action in subcutaneous tissues.
Dosage and Administration Guidelines
- Standard Dosing: Type 1 diabetics typically inject immediately before meals; type 2 diabetics may receive customized dosing based on glucose trends.
- Meal Timing: Ideally injected within 15 minutes before or immediately after a meal.
- Titration: Adjusted based on frequent capillary glucose monitoring or continuous glucose monitoring data.
- Transitioning: Patients switching from other prandial insulins should undergo close monitoring for dose equivalency and effect.
- Missed Doses: Skipped doses should not be doubled; if a meal is skipped, the corresponding dose should be omitted.

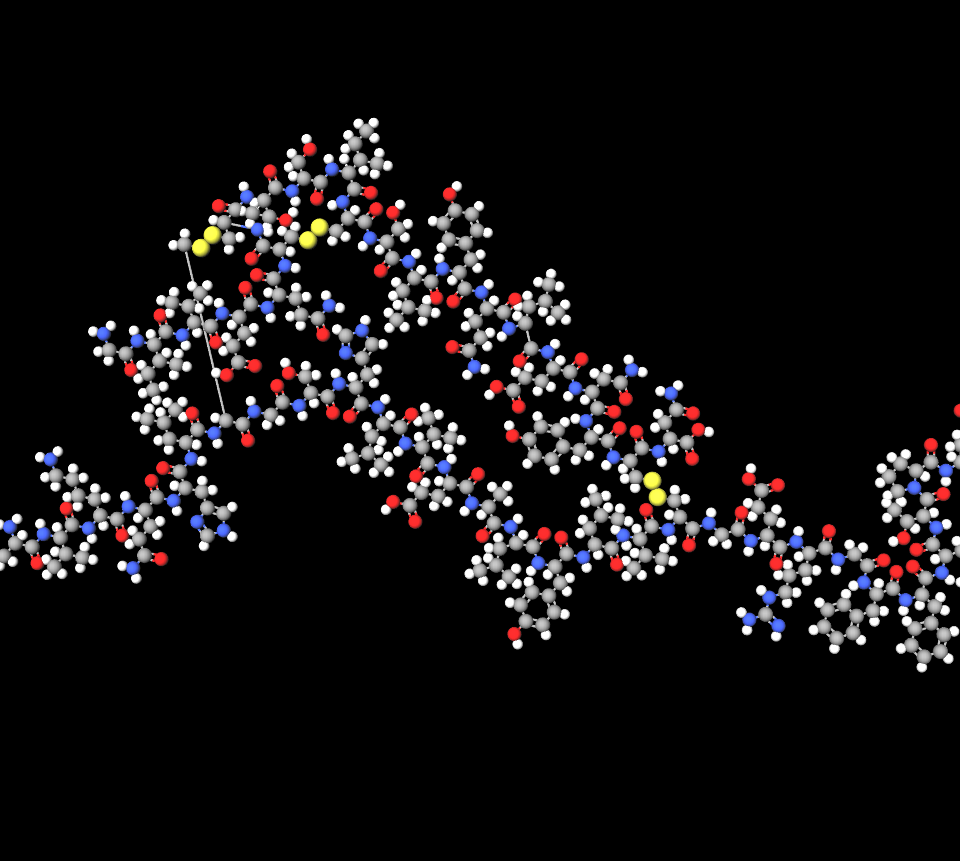
Composition and Formulation Details
- Active Ingredient: Insulin glulisine, 100 units/mL.
- Excipients: Metacresol, sodium chloride, polysorbate 20, tromethamine, zinc, hydrochloric acid, and water for injection.
- Delivery System: Solostar pen is a disposable, dial-a-dose device calibrated to deliver in 1-unit increments up to 80 units per injection.
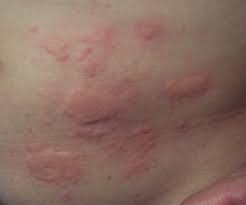
Common and Serious Side Effects
7.1 Common Side Effects of Apidra Solostar
- Localized injection site reactions including erythema, swelling, or lipodystrophy.
- Hypoglycemia, the most frequent adverse effect, is especially when meals are delayed or missed.
- Mild allergic reactions present as rash or pruritus.
7.2 Serious or Rare Side Effects
- Severe hypoglycemia with neuroglycopenic symptoms, including disorientation or seizure.
- Electrolyte disturbances, such as hypokalemia, can trigger arrhythmias in susceptible patients.
- Systemic hypersensitivity or anaphylaxis, though rare, necessitating emergency intervention.
Drug Interactions and Contraindicated Medications
- Beta-Blockers: May mask hypoglycemia symptoms, complicating glucose monitoring.
- Corticosteroids and Diuretics: Can antagonize insulin's glucose-lowering effect.
- Alcohol: Increases risk of delayed hypoglycemia, especially with fasting or irregular meals.
- Thiazolidinediones: Co-administration may lead to fluid retention and exacerbate heart failure.
- Contraindicated Drugs: Avoid use with MAO inhibitors and certain ACE inhibitors due to increased hypoglycemia risk.
Warnings and Precautions for Safe Use
- Hypoglycemia Awareness: Patients must be trained to identify and correct low blood sugar promptly.
- Impaired Hepatic or Renal Function: Increased sensitivity to insulin may necessitate dose adjustments.
- Pen Operation: Requires sufficient visual acuity and manual dexterity; not suitable for blind or arthritic patients unless assisted.
- Needle Reuse: Discouraged due to infection risk and needle dullness that may lead to tissue trauma.
Contraindications to Apidra Solostar Use
- Hypersensitivity Reactions: Individuals with a known allergy to insulin glulisine or any of the excipients in Apidra Solostar should strictly avoid its use. Manifestations may include urticaria, bronchospasm, or anaphylaxis.
- Ongoing Hypoglycemia: Apidra should not be administered during an active episode of low blood sugar, as it may exacerbate hypoglycemic symptoms and pose a life-threatening risk.
- Critical Care Needs: In patients requiring intravenous insulin such as during diabetic ketoacidosis (DKA), surgery, or intensive care subcutaneous Apidra is not appropriate and should be replaced with intravenous insulin regimens.

Guidelines for Careful Administration
- Injection Site Rotation: Rotate sites such as the abdomen, thigh, or upper arm to prevent lipohypertrophy or localized tissue fibrosis.
- Blood Glucose Monitoring: Maintain rigorous documentation of fasting and postprandial glucose levels to fine-tune dosage and timing.
- Initiation in New Patients: For insulin in need individuals, start with conservative dosing under clinical supervision to avoid hypoglycemia.
- Low-Monitoring Environments: Use with caution where access to regular glucose monitoring is limited. Patients should be educated to recognize hypoglycemia symptoms without reliance on glucometers.
Important Precautions Before and During Use
- Travel Considerations: Plan for time zone changes to maintain consistent dosing schedules. Carry backup insulin, glucometers, and medical identification.
- Temperature Protection: Avoid exposure to freezing temperatures or direct sunlight. Carry in temperature-controlled pouches during transport.
- Family Education: Train household members or caregivers to recognize hypoglycemia and administer rescue glucose therapy if needed. Include instruction on glucagon use where applicable.
Storage and Handling Requirements
- Before Opening: Store unopened pens in a refrigerator between 2°C to 8°C. Do not freeze.
- After Opening: Once in use, store at room temperature (below 25°C) and discard after 28 days, even if some insulin remains.
- Maintaining Efficacy: Do not expose to light, excessive vibration, or prolonged heat. Visually inspect for discoloration or particulate matter before injection.
- Disposal of Sharps: Used needles and pens should be disposed of in FDA-cleared sharps containers. Never throw loose needles into household trash.
Use in Special Populations
14.1 Administration to Elderly Patients
- Renal Function Considerations: Reduced renal clearance in older adults may necessitate lower starting doses.
- Fall Risk: Increased susceptibility to hypoglycemia-related confusion or dizziness elevates the risk of falls and injuries.
- Polypharmacy Surveillance: Monitor closely for interactions with antihypertensives, diuretics, and other medications common in geriatric regimens.

14.2 Use in Pregnant Women and Nursing Mothers
- Pregnancy Safety: Classified as Category B; animal studies have not demonstrated fetal risk, but human studies are limited.
- Monitoring Needs: Pregnancy-induced insulin resistance requires ongoing dose adjustments and close obstetric-endocrinologic collaboration.
- Lactation: Insulin does not pass significantly into breast milk and is generally considered safe during breastfeeding. Monitor neonates for hypoglycemia.
14.3 Use in Pediatric Patients
- Approved Use: Apidra is FDA-approved for use in children aged 4 years and older with type 1 diabetes.
- Sensitivity Variations: Children may have increased insulin sensitivity and fluctuating activity levels, requiring vigilant monitoring.
- Parental Guidance: Educate caregivers in proper injection technique, dose measurement, and recognizing signs of hypo/hyperglycemia in children.
Overdose Risks and Emergency Management
- Warning Signs: Symptoms of insulin overdose include sweating, tremors, confusion, visual disturbances, and seizures. Prolonged episodes may lead to coma.
- Immediate Response: Administer fast-acting carbohydrates (glucose tablets, juice) or glucagon injections for unconscious patients. Contact emergency services immediately.
- Post-Crisis Management: Hospitalization may be required for intravenous dextrose support and continuous monitoring. Reevaluate dosing to prevent recurrence.
Handling and Disposal Precautions
- Sharps Disposal: Adhere to local health authority regulations for disposal. Use puncture-proof containers and avoid makeshift alternatives.
- Pen Maintenance: Clean exterior with a soft, dry cloth. Never immerse in water or use alcohol-based cleaners.
- Pen Sharing Warning: Sharing Solostar pens is strictly contraindicated, even when the needle is changed, due to the risk of bloodborne pathogen transmission.
- Label Tracking: Always label pens with the start date and verify patient identity before each use to prevent dosing errors.
Apidra Solostar Pen-Injection FAQ
- When is the best time to inject Apidra?
- What is Apidra SoloStar used for?
- How to inject Apidra pen?
- How many units of Apidra should I take?
- What is the duration of Apidra SoloStar?
- Does Apidra need to be refrigerated?
- What are the side effects of Apidra insulin?
- How long does it take for Apidra to start working?
- Can I take Apidra after a meal?
- What are the benefits of Apidra?
- How many ml is an Apidra pen?
- What is the difference between Apidra and regular insulin?
- What type of insulin is glulisine?
- Is insulin glulisine rapid?
- Is insulin glulisine long or short acting?
- What is the difference between insulin aspart and insulin glulisine?
- What is insulin glulisine used for?
- What is Apidra SoloStar?
- How does Apidra SoloStar work?
- Who typically uses Apidra SoloStar?
- How is Apidra SoloStar administered?
- When is Apidra SoloStar typically injected?
- What are the common side effects of Apidra SoloStar?
- How should Apidra SoloStar be stored?
- Can Apidra SoloStar be used with other insulins?
- What should I do if I miss a dose of Apidra SoloStar?
When is the best time to inject Apidra?
Apidra assists in regulating your blood sugar levels during meals, allowing you to administer it up to 15 minutes before or within 20 minutes after beginning a meal.
What is Apidra SoloStar used for?
Apidra is a type of rapid-acting insulin used by both adults and children with diabetes to manage blood sugar levels.
How to inject Apidra pen?
Ensure that you prepare the insulin and attach the needle securely before each use to maintain sterility and safety standards. Before administering the injection dose, remember to conduct a safety test as a measure. Adjust the dosage as needed in increments of 1 unit, between 1 and 80 units, based on your requirements. Once ready, proceed to inject the dose as instructed.
How many units of Apidra should I take?
0.5 to 1 Unit/kg/day
What is the duration of Apidra SoloStar?
4 hours
Does Apidra need to be refrigerated?
No
What are the side effects of Apidra insulin?
Low blood sugar (hypoglycemia), allergic reactions (such as itching and rash), and pain or redness alongside itching or swelling at the site of the injection or infusion are some symptoms to watch out for.
How long does it take for Apidra to start working?
15 minutes after injection
Can I take Apidra after a meal?
It is recommended to administer Apirdra 15 minutes right after having a meal.
What are the benefits of Apidra?
To control high blood sugar
How many ml is an Apidra pen?
Each pen comes with 3 milliliters of injection solution, which is the same as 300 Units.
What is the difference between Apidra and regular insulin?
Apidra(R), in contrast to insulin, starts working quickly and has a shorter period of effectiveness.
What type of insulin is glulisine?
Insulin glulisine, a rapid-acting analogue of insulin, works more quickly. For a shorter time than regular human insulin in people with type 1 or type 2 diabetes, effectively managing meal-related blood sugar levels in these individuals.
Is insulin glulisine rapid?
Yes
Is insulin glulisine long or short acting?
Short-acting
What is the difference between insulin aspart and insulin glulisine?
Aspart has shown physical stability in the insulin pump when compared to lispro and glulisine. It also has the lowest rates of occlusion overall.
What is insulin glulisine used for?
Insulin glulisine is prescribed for managing type 1 diabetes, a health condition where the body lacks insulin production leading to difficulty, in regulating blood sugar levels.
What is Apidra SoloStar?
Apidra SoloStar is a pen injector filled with Apidra (insulin glulisine), a working insulin similar to those used to reduce blood sugar levels in adults and children with diabetes.
How does Apidra SoloStar work?
Apidra (insulin glulisine) works by aiding the transfer of glucose (or sugar) from the bloodstream to body tissues for energy utilization and impedes excessive glucose production in the liver. Being fast in action, it begins to take effect post-injection within a span of 10 to 20 minutes.
Who typically uses Apidra SoloStar?
Individuals with type 1 diabetes and adults with type 2 diabetes who need insulin to control their blood sugar levels often use it during meals and with insulin pumps as well.
How is Apidra SoloStar administered?
Apidra SoloStar is administered by injecting it under the skin in places such as the stomach area, thigh, or upper arm region.
When is Apidra SoloStar typically injected?
Apidra SoloStar should typically be injected before or shortly after a meal to quickly manage the increase in blood sugar following eating.
What are the common side effects of Apidra SoloStar?
The primary side effect often observed is hypoglycemia, or low blood sugar levels, accompanied by side effects such as injection site reactions (including redness, swelling, and itching), allergic responses, and lipodystrophy (alterations in fat tissue at the injection site, particularly when the same site is used repeatedly).
How should Apidra SoloStar be stored?
Remember to keep any Apirda SoloStar pens in the fridge (between 36°F and 46°F) and shielded from light until use. After opening it for use you can store the pen at room temperature (Up to 86°F) for 28 days without needing refrigeration anymore. It's essential not to freeze the pen and to discard it if it has become frozen at any point.
Can Apidra SoloStar be used with other insulins?
Apidra SoloStar can be effectively incorporated into a basal insulin plan alongside long-acting insulins; just remember not to mix different insulin types in the same syringe or pen.
What should I do if I miss a dose of Apidra SoloStar?
If you happen to skip a mealtime dose of Apidra SoloStar and find your blood sugar levels elevated, you may require a dose for correction purposes. Nevertheless, it's important to keep a check on your blood sugar and seek guidance from your healthcare provider regarding how to handle missed doses.