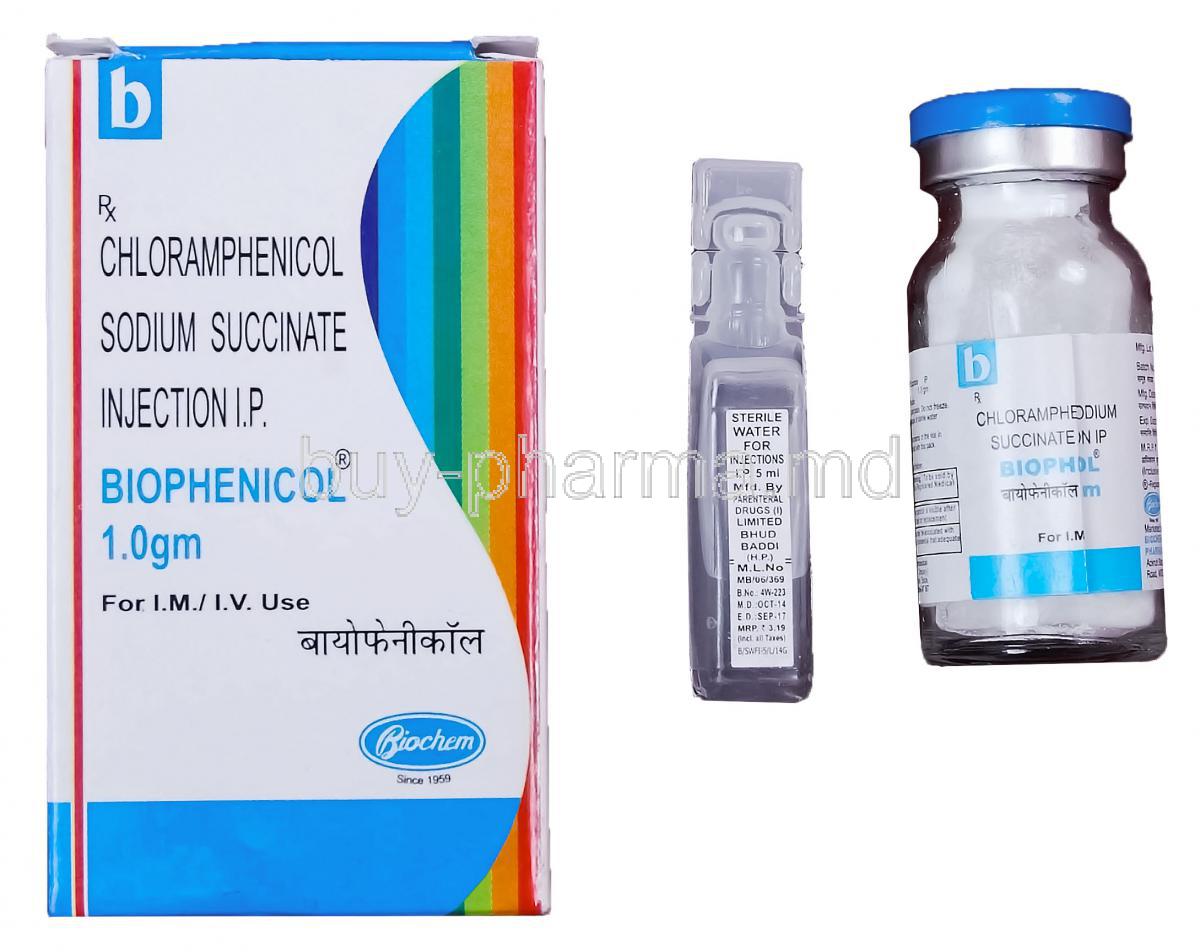
Chloramphenicol Injection
- Introduction to Chloramphenicol Injection
- Composition and Formulation Details
- Therapeutic Uses of Chloramphenicol Injection
- Off-Label and Investigational Uses of Chloramphenicol Injection
- Chloramphenicol mechanism of action
- Chloramphenicol dosage
- Chloramphenicol side effects
- Chloramphenicol interactions
- Warnings and Precautions Before and During Treatment
- Contraindications for Chloramphenicol Injection Use
- Guidelines for Careful Administration in Special Populations
- Overdose and Emergency Management
- Chloramphenicol storage
- Handling and Disposal Precautions for Healthcare Providers
Introduction to Chloramphenicol Injection
Composition and Formulation Details
Chloramphenicol drug class
Chloramphenicol acetyltransferase
Chloramphenicol working concentration
Therapeutic Uses of Chloramphenicol Injection
Off-Label and Investigational Uses of Chloramphenicol Injection
Chloramphenicol mechanism of action
Chloramphenicol dosage
Chloramphenicol side effects
7.1 Common Side Effects
7.2 Serious Side Effects
Chloramphenicol interactions
Warnings and Precautions Before and During Treatment
Contraindications for Chloramphenicol Injection Use
Guidelines for Careful Administration in Special Populations
11.1 Administration to Elderly Patients
11.2 Use in Pregnant Women and Nursing Mothers
11.3 Use in Pediatric Populations
Overdose and Emergency Management
Chloramphenicol storage
Handling and Disposal Precautions for Healthcare Providers
Chloramphenicol Injection FAQ
- What is chloramphenicol injection used for?
- Can chloramphenicol be given IV?
- What is chlorpromazine injection used for?
- Can chloramphenicol be used to treat wounds?
- Is chloramphenicol a strong antibiotic?
- What type of bacteria is killed by chloramphenicol?
- Who Cannot use chloramphenicol?
- What is chloramphenicol used against?
- What is the brand name for chloramphenicol IV?
- Is chlorpromazine given IV or IM?
- What type of infection does chloramphenicol treat?
- What bacteria is resistant to chloramphenicol?
- What are the side effects of chloramphenicol?
- Can I buy chloramphenicol over the counter?
- What are the hazards of chloramphenicol?
What is chloramphenicol injection used for?
Chloramphenicol injection is prescribed for treating infections caused by bacteria in cases where alternative antibiotics are not viable options.
Can chloramphenicol be given IV?
Chloramphenicol needs to be given through a vein in a 10 percent solution (100 mg/ml) or at a concentration.
What is chlorpromazine injection used for?
Chlorpromazine is a drug that is prescribed to help control and address conditions such as schizophrenia and bipolar disorder, as well as acute psychotic episodes. It falls under the group of antipsychotics or neuroleptic medications. It is sometimes referred to as a first-generation antipsychotic.
Can chloramphenicol be used to treat wounds?
Chloramphenicol ointment is recommended for treating conjunctivitis; however, there is evidence to support its use in preventing or treating wound infections.
Is chloramphenicol a strong antibiotic?
Chloramphenicol is able to combat a range of microorganisms; however, its usage in humans is limited to treating life-threatening infections due to its potential serious side effects, such as bone marrow damage, leading to conditions like aplastic anemia or typhoid fever treatment purposes.
What type of bacteria is killed by chloramphenicol?
Chloramphenicol is an antibiotic that works against a range of bacteria, including gram-negative types, spirochetes, and Rickettsiae.
Who Cannot use chloramphenicol?
Anyone suffering from aplastic anaemia (when the bone marrow does not produce blood cells)
What is chloramphenicol used against?
Chloramphenicol is a medicine commonly prescribed to treat eye infections, like conjunctivitis and otitis externa. It is also effective against typhoid and cholera infections due to its antibiotic properties that target protein synthesis inhibition.
What is the brand name for chloramphenicol IV?
Chloroptic, AK-Chlor, Chloromycetin Ophthalmic, Ocu-Chlor, Chloroptic S.O.P.
Is chlorpromazine given IV or IM?
Administer intravenously as a 10 percent (100 milligrams per milliliter) solution over a period of at least one minute following the mixing of 10 milliliters of a liquid diluent like sterile Water for Injection or 5 percent Dextrose for Injection.
What type of infection does chloramphenicol treat?
Chloramphenicol functions by disrupting the production of proteins for growth and reproduction in the body. It's a common treatment for typhoid fever, other Salmonella infections, rickettsial infections, and meningitis
What bacteria is resistant to chloramphenicol?
Pseudomonas putida KT2440
What are the side effects of chloramphenicol?
This medication could lead to side effects such as issues with blood and eyesight. Signs of blood problems may include having skin that is redder than usual or experiencing a sore throat and fever; your body might also show signs of unusual bleeding or bruising and unexplained fatigue or weakness.
Can I buy chloramphenicol over the counter?
Yes
What are the hazards of chloramphenicol?
It can lead to eye injury, potential cancer risk, and harm to fertility or unborn children; seek specific guidance before use.