1. Introduction to Himalaya Liv.52 HB
Himalaya Liv.52 HB is a specialized hepatoprotective formulation developed for individuals with chronic liver conditions, especially those infected with the hepatitis B virus. Designed using a blend of potent Ayurvedic herbs, this formulation aims to restore, protect, and enhance liver health through natural therapeutic mechanisms.
Unlike the standard Liv.52 and Liv.52 DS variants, Liv.52 HB is tailored for hepatological conditions associated with viral infections, offering a unique spectrum of benefits. It is specifically recommended for patients diagnosed with hepatitis B, liver fibrosis, and related degenerative hepatic conditions.
The product integrates traditional Ayurvedic wisdom with modern phytotherapeutic research to deliver clinically validated support for liver recovery and function.
2. Composition and Active Herbal Ingredients
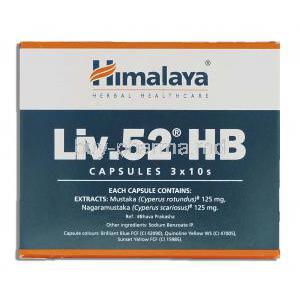
Liv.52 HB contains a synergistic blend of herbal extracts, each selected for their hepatoprotective, anti-inflammatory, and detoxifying properties:
- Himsra (Capparis spinosa): Known for its ability to protect liver cells from chemical-induced damage and promote hepatocyte regeneration.
- Kasani (Cichorium intybus): A bitter tonic that enhances bile secretion and supports liver detoxification.
- Kakamachi (Solanum nigrum): Offers anti-inflammatory and antioxidant support while stabilizing liver enzyme levels.
- Arjuna (Terminalia arjuna): Reinforces hepatic circulation and acts as a free radical scavenger.
- Kasamarda (Cassia occidentalis): Traditionally used in Ayurveda to detoxify the liver and support immune function.
- Mandur Bhasma (Iron Calx): A processed form of iron used to combat hepatic anemia and improve liver vitality.
This polyherbal synergy enhances the formula’s efficacy in hepatoprotection, detoxification, and immunomodulation.
3. Mechanism of Action: How Himalaya Liv.52 HB Works
Liv.52 HB exerts its therapeutic action through multiple biological pathways:
- Antioxidant and Anti-inflammatory Effects: Neutralizes reactive oxygen species and inhibits inflammatory cascades that damage hepatocytes.
- Hepatocellular Regeneration: Stimulates DNA and protein synthesis in liver cells, promoting faster tissue repair.
- Hepatotoxin Neutralization: Prevents liver damage from chemicals, drugs, and alcohol by stabilizing cell membranes.
- Liver Enzyme Regulation: Normalizes elevated transaminases and improves overall liver enzyme profile.
- Immune Support: Modulates immune responses in chronic hepatitis B, helping control viral activity and liver inflammation.
4. Therapeutic Uses of Himalaya Liv.52 HB
4.1 Primary Indications
- Supportive therapy in hepatitis B infection
- Adjunct treatment for liver cirrhosis and hepatic fibrosis
- Facilitates recovery of liver function post-viral hepatitis
4.2 Off-label and Supportive Uses
- Liver protection in individuals consuming alcohol regularly
- Supportive therapy in non-alcoholic fatty liver disease (NAFLD)
- Helps prevent liver damage from hepatotoxic drugs such as anti-tuberculars or chemotherapy agents
- Hepatic support in metabolic syndrome, insulin resistance, and type 2 diabetes
5. Recommended Dosage and Administration Guidelines
Liv.52 HB is available in both tablet and syrup forms, ensuring flexibility for different patient needs:
- Adults: Typically 1–2 tablets twice daily or 10 ml syrup twice daily
- Duration: As prescribed, often continued for several weeks or months in chronic conditions
- With or without food: Preferably taken before meals to optimize absorption
If a dose is missed, it should be taken as soon as remembered unless it's close to the next scheduled dose. Doubling the dose should be avoided.
6. Common and Rare Side Effects of Liv.52 HB
6.1 Frequently Reported Side Effects
- Mild gastrointestinal upset such as nausea or diarrhea
- Headache or lightheadedness, typically transient
6.2 Rare but Serious Adverse Reactions
- Hypersensitivity reactions including rash, itching, or swelling
- Exacerbation of autoimmune diseases (rare, theoretical concern)
- Iron overload symptoms in individuals with iron metabolism disorders
7. Drug and Herbal Interactions
- May interact with antiviral medications like tenofovir and entecavir
- Use caution when taken with corticosteroids or immunosuppressive therapy
- Combined use with other hepatotonics or herbal formulations should be monitored
- Alcohol should be avoided to reduce cumulative liver burden
8. Important Warnings and Safety Information
- Liv.52 HB is not a replacement for prescription antiviral therapy in hepatitis B
- Patients with diagnosed liver disease should use only under medical supervision
- Improper or prolonged use may obscure serious liver conditions requiring urgent care
9. Contraindications: When Not to Use Liv.52 HB
- Allergy or hypersensitivity to any of the herbal components
- Patients with iron storage disorders such as hemochromatosis or hemosiderosis
- Individuals with severe renal impairment due to iron content
- Simultaneous use with other hepatoprotective agents without clinician guidance
10. Careful Administration and Monitoring Guidelines
Long-term administration of Himalaya Liv.52 HB necessitates a structured approach to monitoring and dosing. Routine liver function tests (LFTs) are essential to evaluate the efficacy of treatment and detect any emerging hepatic abnormalities. These tests help in tracking parameters such as ALT, AST, bilirubin, and albumin levels.
For individuals with heightened sensitivity—whether due to gastrointestinal issues, metabolic concerns, or underlying health conditions—initiating treatment with a lower dose is advisable. This gradual escalation minimizes the risk of intolerance and improves adherence.
Patients undergoing polypharmacy, particularly those taking antivirals, antihypertensives, or immunosuppressants, should be carefully monitored. Herb-drug interactions, while uncommon, may alter therapeutic outcomes or potentiate adverse effects.
Caution is strongly warranted in individuals with autoimmune hepatitis, as immune modulation from herbal components may exacerbate inflammatory responses. A hepatologist’s input is critical in such cases.
11. Special Precautions Before and During Use
During the course of Liv.52 HB therapy, patients must remain vigilant for any signs of adverse reactions. Symptoms such as unexplained fatigue, jaundice, or gastrointestinal distress should prompt immediate medical review and potential discontinuation.
Maintaining optimal hydration and adhering to a liver-supportive diet—low in saturated fats, refined sugars, and alcohol—is strongly advised. Nutritional support synergizes with the supplement’s effects and supports hepatic regeneration.
Liv.52 HB should not be considered a monotherapy for chronic hepatitis B or other serious liver diseases. It functions best as an adjunctive therapy alongside standard antiviral or hepatologic care.
Concurrent consumption of alcohol or use of hepatotoxic drugs must be avoided, as these can undermine therapeutic outcomes and elevate hepatic stress.
12. Administration in Special Populations
12.1 Elderly Patients
Aging is often accompanied by diminished hepatic perfusion and metabolic capacity. Therefore, dose adjustments may be necessary in elderly individuals to account for reduced hepatic clearance.
Given the prevalence of polypharmacy in this population, there is an elevated risk of herb-drug interactions. Detailed medication reconciliation and periodic monitoring are essential for safety.
12.2 Pregnant and Nursing Women
There is currently insufficient clinical data to support the use of Liv.52 HB during pregnancy. Although the formulation is plant-based, the presence of iron (Mandur Bhasma) and certain potent botanicals warrants caution due to unknown fetal effects.
During lactation, use is generally discouraged unless explicitly prescribed. Transfer of active constituents through breast milk and their impact on neonates remain inadequately studied.
12.3 Pediatric Use
Liv.52 HB should only be administered to children under strict pediatric supervision. Safety and efficacy in the pediatric population have not been conclusively established.
When prescribed, dosing should be weight-adjusted and administered in syrup form for easier compliance. Pediatric use should be limited to conditions clearly benefiting from hepatoprotective therapy.
13. Overdose and Toxicity Management
In the event of overdose, symptoms such as abdominal discomfort, iron overload, nausea, or vomiting may manifest. Excessive intake of Mandur Bhasma can also pose oxidative risks to organs.
Immediate action involves cessation of the product and symptomatic management. Induced vomiting or gastric lavage may be considered in acute ingestion cases under clinical supervision.
There is no known specific antidote for Liv.52 HB. Emergency care should be sought to stabilize vital signs, perform blood tests, and manage systemic complications.
14. Storage Conditions and Stability
- Temperature: Store at 15–25°C in a cool, dry place.
- Humidity: Keep away from damp environments to prevent tablet degradation and microbial contamination.
- Light: Avoid exposure to direct sunlight or high temperatures to preserve potency.
- Shelf-life: Observe expiry date on packaging; avoid use of expired formulations.
- Child safety: Always keep out of reach of children to prevent accidental ingestion.
15. Handling and Disposal Precautions
When handling Liv.52 HB tablets or syrup, ensure hands are clean and dry to prevent contamination. The syrup cap should be tightly secured after each use to maintain integrity.
Tablets should not be crushed or altered unless directed by a healthcare provider, as doing so may affect absorption and efficacy.
Expired or unused products should be disposed of in accordance with local pharmaceutical disposal regulations. Avoid flushing into drains or throwing in household trash.
As an herbal formulation, environmental care is warranted to prevent ecological impact from bulk disposal. Consult with local pharmacies or waste disposal authorities for safe practices.