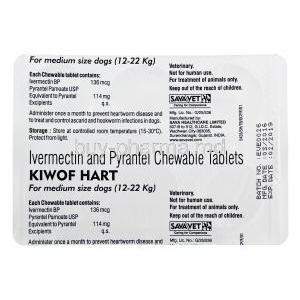
Introduction to Kiwof Hart Chewable Tab
Overview of Kiwof Hart as a Veterinary Deworming Product
Kiwof Hart Chewable Tab is a broad-spectrum anthelmintic formulated specifically for canines. Designed to eliminate and prevent various internal parasitic infections, this chewable tablet offers both convenience and effectiveness. - Palatable chewable format for easy administration - Monthly treatment regimen supports long-term control
Importance of Routine Deworming in Canine Health
Internal parasites such as heartworms, roundworms, and hookworms pose a significant threat to canine wellness. Without routine prophylactic measures, dogs may suffer from: - Lethargy and weight loss - Gastrointestinal upset - Severe cardiopulmonary complications Routine deworming is crucial to break the lifecycle of parasites and prevent reinfestation.
Manufacturer and Regulatory Approvals
Kiwof Hart is manufactured under strict quality assurance protocols by Sava Vet, a trusted name in veterinary pharmaceuticals. The formulation complies with veterinary health guidelines and is approved for distribution in multiple regulated markets.
Composition and Active Ingredients
Detailed Breakdown of Active Components: Ivermectin and Pyrantel Pamoate
Each chewable tablet of Kiwof Hart contains: - **Ivermectin** – 6 mcg/kg body weight - **Pyrantel Pamoate** – 14.4 mg/kg body weight These actives target both larval and adult stages of parasites.
Role of Each Ingredient in Parasite Control
- **Ivermectin** disrupts the nervous system of microfilariae (heartworm larvae), causing paralysis and death. - **Pyrantel Pamoate** acts as a depolarizing neuromuscular blocker in nematodes, leading to expulsion from the gastrointestinal tract.
Inactive Ingredients and Formulation Specifics
The formulation includes flavoring agents, binders, and excipients to enhance palatability and stability. It is free from artificial preservatives and commonly recognized allergens.
Mechanism of Action: How Kiwof Hart Works
Ivermectin: Paralysis and Death of Heartworm Larvae
Ivermectin increases the release of gamma-aminobutyric acid (GABA) in parasite neurons, leading to neuromuscular paralysis. This results in the elimination of **Dirofilaria immitis** larvae before they mature into adult worms.
Pyrantel Pamoate: Neuromuscular Blockade in Intestinal Worms
Pyrantel functions by depolarizing the nerve endings of parasitic worms. The muscle contraction is sustained, paralyzing the worm, which is then excreted via feces.
Combined Spectrum of Activity Against Internal Parasites
Together, the actives deliver broad-spectrum efficacy against: - Heartworms - Roundworms - Hookworms This synergistic action ensures optimal protection with a single monthly dose.
Approved Uses and Therapeutic Indications
Prevention of Heartworm Disease in Dogs
Kiwof Hart is indicated for the **prevention of canine heartworm disease** when administered monthly. It inhibits early-stage larval development before cardiac complications can occur.
Treatment and Control of Roundworms
Effective against: - *Toxocara canis* - *Toxascaris leonina* Prevents intestinal blockage and malnutrition caused by nematode infestations.
Treatment and Control of Hookworms
Targets: - *Ancylostoma caninum* - *Uncinaria stenocephala* Reduces risk of blood loss anemia and gastrointestinal distress.
Off-Label and Additional Uses
Off-Label Use in Treatment of Whipworms or Other Parasitic Infestations
Veterinarians may prescribe Kiwof Hart off-label for control of other helminths, depending on regional parasite prevalence and susceptibility.
Use in Mixed Parasitic Infestations
Especially useful in shelter environments or rescues, where dogs may harbor multiple parasites simultaneously.
Dosage and Administration Guidelines
Recommended Dosage Based on Dog’s Weight
- Dogs up to 11 kg: 1 tablet - 12–22 kg: 2 tablets - 23–45 kg: 3 tablets Administer monthly for sustained efficacy.
Chewable Tablet Administration: With or Without Food
Can be administered directly or hidden in food. Most dogs accept it as a treat due to its palatable flavor.
Monthly Dosing Schedule and Timing
To prevent heartworm, maintain a strict 30-day interval. Ideally, administer on the same calendar date each month.
Missed Dose Instructions
If a dose is missed by more than 30 days, consult a veterinarian before resuming. Delays increase heartworm infection risk.
Side Effects and Adverse Reactions
Overview of Potential Side Effects in Dogs
Most dogs tolerate Kiwof Hart well. However, some may experience mild to moderate adverse effects.
Frequency and Severity of Observed Reactions
- Common: transient gastrointestinal symptoms - Rare: neurological signs in susceptible breeds - Very rare: hypersensitivity reactions in microfilaria-positive dogs
Rare or Serious Adverse Events
Serious events are infrequent but may include seizures or anaphylaxis in dogs with heavy parasite burdens.
Common Side Effects in Dogs
Gastrointestinal Symptoms
- Vomiting - Diarrhea - Flatulence These symptoms typically resolve within 24–48 hours without intervention.
Neurological Symptoms
- Ataxia - Tremors - Disorientation Observed primarily in MDR1 mutation-carrying breeds.
Lethargy and Reduced Appetite
Dogs may appear subdued or eat less within 12 hours post-dosing. Monitoring is advised.
Drug Interactions and Compatibility
Known Drug Interactions
Use cautiously with: - Other macrocyclic lactones - Immunosuppressants Combined use may elevate neurotoxic risk.
Cautions with Concurrent Vaccinations or Treatments
Avoid administering Kiwof Hart on the same day as live vaccines or other systemic treatments unless approved by a vet.
Veterinary Consultation for Multi-Drug Regimens
Consultation ensures safe polypharmacy, especially in chronically ill or elderly dogs.
Warnings and Safety Considerations
Safety Margin in Different Breeds and Sizes
Kiwof Hart has a wide safety margin but should be dose-adjusted precisely for small and toy breeds.
Genetic Sensitivity in Certain Breeds
Breeds such as Collies, Australian Shepherds, and Shetland Sheepdogs may have a genetic mutation (MDR1) making them more susceptible to ivermectin toxicity.
Risk of Toxicity in Overdose Situations
Symptoms of overdose include: - Hypersalivation - Coma - Respiratory distress Immediate veterinary intervention is critical.
Contraindications for Use
Dogs with a History of Hypersensitivity
Avoid use in animals with known allergies to ivermectin or pyrantel.
Use in Sick or Debilitated Animals
Not recommended unless a veterinarian determines benefit outweighs risk.
Not Indicated for Use in Cats or Other Non-Canine Species
Species-specific pharmacodynamics make this product unsuitable for non-canine use.
Special Populations and Careful Administration
Use in Elderly Dogs
- Start at lowest effective dose - Monitor liver and renal function Older dogs may have slower metabolic clearance.
Risk-Benefit Evaluation in Age-Related Organ Impairment
Liver or kidney dysfunction increases risk of adverse events. Adjust therapy accordingly.
Use in Pregnant or Lactating Dogs
- Limited data on reproductive safety - Avoid during early gestation unless advised by a vet Pyrantel is generally regarded as safer than ivermectin in pregnancy.
Veterinary Discretion in Breeding Females
Routine risk assessment is necessary prior to administration during mating, gestation, or lactation.
Use in Puppies and Juvenile Dogs
- Safe for puppies over 6 weeks of age and 2 kg body weight - Dosing must align with weight gain during growth phase Parasite load is typically heavier in young dogs, requiring vigilant monitoring.
Overdose and Toxicity Management
Signs of Ivermectin or Pyrantel Overdose
An overdose of Kiwof Hart Chewable Tab may result in a constellation of alarming symptoms, particularly in dogs with low tolerance thresholds. Clinical manifestations typically include: - Hypersalivation or excessive drooling - Ataxia, tremors, or muscle rigidity - Mydriasis (pupil dilation) - Disorientation or stupor - Vomiting and persistent diarrhea - Respiratory depression in severe cases Neurotoxicity is the principal concern with ivermectin overdoses, while gastrointestinal disturbances are more common with pyrantel excess.
Emergency Response Measures
Immediate veterinary intervention is paramount in suspected overdose cases. Timely management can mitigate systemic toxicity and prevent irreversible damage. Initial at-home steps before veterinary arrival may include: - Removing access to the product - Monitoring for sudden collapse or seizures - Preventing aspiration if vomiting occurs - Keeping the animal warm and calm Owners should never attempt to induce vomiting or administer human medications.
Veterinary Detoxification Protocols
At the clinic, detoxification may involve a multi-modal approach: - Activated charcoal administration (within a narrow therapeutic window) - Intravenous fluid therapy to support renal clearance - Anticonvulsants (e.g., diazepam or phenobarbital) in case of seizures - Oxygen supplementation if respiratory suppression is noted - Hospitalization and continuous observation for up to 48 hours In MDR1-sensitive breeds, genomic testing may be advised post-recovery.
Important Precautions for Use
Necessity of Prior Heartworm Testing Before Initiation
Before administering Kiwof Hart, it is imperative to confirm that the dog is not already infected with adult heartworms. In infected dogs, sudden die-off of microfilariae can trigger severe hypersensitivity reactions or thromboembolic events. A blood test (antigen or microfilaria test) should be performed under veterinary supervision.
Not a Treatment for Adult Heartworms
Kiwof Hart is a prophylactic agent—it halts the maturation of early-stage heartworm larvae. It does not eliminate adult *Dirofilaria immitis*. In dogs with established heartworm disease, a specialized adulticide regimen is required under veterinary care.
Adherence to Monthly Schedule to Prevent Resistance
Skipping doses or irregular administration increases the risk of drug resistance and therapeutic failure. Continuous monthly administration: - Maintains consistent plasma drug levels - Prevents reinfestation - Supports overall parasite control in the community A digital calendar or medication reminder is recommended to ensure compliance.
Storage and Shelf-Life Information
Recommended Storage Temperature and Conditions
Kiwof Hart tablets should be stored in a cool, dry location away from direct sunlight and moisture. Ideal storage temperature: - Between 15°C to 25°C (59°F to 77°F) - Avoid refrigeration or freezing - Store in original blister packaging until use
Shelf-Life and Expiry Date Considerations
Each batch of Kiwof Hart comes with a clearly marked expiry date. - Do not administer tablets past their expiration. - Potency and safety cannot be guaranteed post-expiry. Regular inventory checks are advised, especially in multi-pet households or clinics.
Safe Disposal of Unused or Expired Tablets
Do not flush unused tablets or discard in household trash. - Consult local veterinary pharmacies or pet health services for medication disposal programs - Wrap tablets in a sealed container or bag to prevent accidental ingestion by animals or children if disposal via garbage is unavoidable
Handling Instructions and Owner Safety
Guidelines for Safe Administration to Dogs
- Administer the chewable tablet directly into the dog’s mouth or mix with a small portion of food - Ensure the entire dose is consumed—observe the dog until ingestion is complete - Do not split or crush the tablet unless directed
Hygiene Recommendations After Handling Tablets
After administering Kiwof Hart: - Wash hands thoroughly with soap and water - Avoid touching the eyes, mouth, or other mucosal surfaces until hands are clean - Wear gloves if administering to multiple dogs or handling doses frequently
Keeping Product Out of Reach of Children and Other Pets
- Store in a locked cabinet or pet-inaccessible drawer - Do not leave tablets unattended on counters or in open containers - Child-resistant packaging should remain intact until use Proper storage and handling not only ensure the effectiveness of the medication but also protect household safety.