Strongheart Plus Chewable
- Introduction to Strongheart Plus Chewable
- Active Ingredients and Composition
- Approved Veterinary Uses of Strongheart Plus
- Off-Label and Investigational Uses in Veterinary Medicine
- Mechanism of Action and Pharmacodynamics
- Dosage Guidelines and Administration Instructions
- Storage, Shelf Life, and Handling Precautions
- Side Effects and Tolerance in Dogs
- Most Frequently Reported Common Side Effects
- Contraindications and Breed Sensitivity
- Drug and Product Interactions
- Special Precautions and Veterinary Warnings
- Careful Administration in High-Risk or Compromised Dogs
- Use in Geriatric Dogs (Elderly Patients)
- Use in Pregnant, Breeding, or Lactating Dogs
- Use in Puppies and Pediatric Dogs
- Overdose Symptoms and Emergency Treatment
- Owner Handling and Safety Guidelines
Introduction to Strongheart Plus Chewable
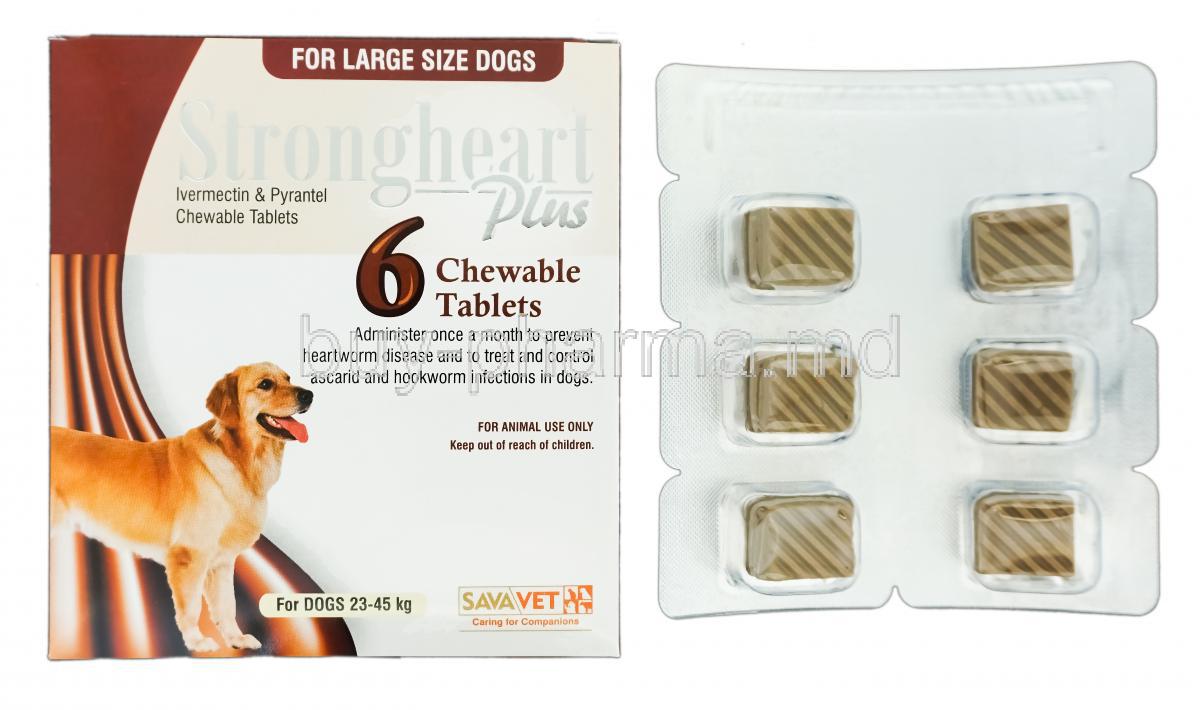
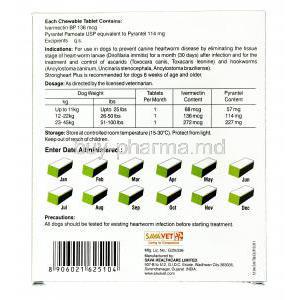
Strongheart Plus Chewable is a widely trusted veterinary medication formulated to prevent heartworm disease in dogs. This palatable, meat-flavored chewable provides broad-spectrum protection against several internal parasites, ensuring year-round defense for companion animals.
Manufactured by a reputable pharmaceutical company specializing in animal health, Strongheart Plus has earned endorsement from veterinarians for its clinical efficacy and ease of administration. Its formulation has been carefully designed to encourage voluntary intake, making it an ideal choice for even the most finicky canine patients.
Active Ingredients and Composition
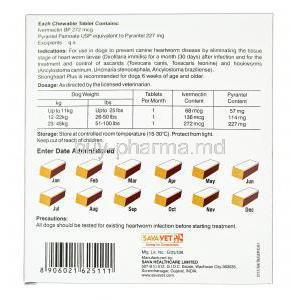
Strongheart Plus Chewable combines two powerful anthelmintic agents:
- Ivermectin: A macrocyclic lactone that targets heartworm larvae (Dirofilaria immitis) during their early developmental stages.
- Pyrantel Pamoate: A depolarizing neuromuscular-blocking agent effective against gastrointestinal nematodes.
Both ingredients are classified within pharmacological categories that address endoparasitic infestations through distinct yet complementary mechanisms. The chewable base also includes inactive ingredients that enhance flavor, texture, and shelf stability.
Strongheart Plus is available in several dosage strengths, each tailored to specific canine weight ranges to ensure accurate and safe dosing.
Approved Veterinary Uses of Strongheart Plus
This medication is approved for the following therapeutic purposes:
- Heartworm prevention: Eliminates the tissue-stage larvae of Dirofilaria immitis before they mature.
- Roundworm control: Effective against Toxocara canis and Toxascaris leonina.
- Hookworm control: Treats and controls Ancylostoma caninum and Uncinaria stenocephala.
- Monthly prophylaxis: Particularly essential for dogs living in or traveling to high-risk, mosquito-prone environments.
Off-Label and Investigational Uses in Veterinary Medicine
Though not officially approved, Strongheart Plus has been explored in the following contexts:
- Occasional use in felines, though efficacy and safety have not been validated through controlled studies.
- Adjunct treatment with other antiparasitics for complex or mixed parasitic infections, subject to veterinary discretion.
- Investigational combination therapies to address emerging resistance patterns or regional parasitic variations.

Mechanism of Action and Pharmacodynamics
Each active ingredient plays a critical role in interrupting parasite life cycles:
- Ivermectin binds to glutamate-gated chloride channels in the nervous system of parasites, inducing paralysis and death by disrupting neurotransmission.
- Pyrantel Pamoate causes spastic paralysis in nematodes by acting as a cholinergic agonist at the parasite's neuromuscular junction.
The synergistic effect leads to rapid onset of action and efficient expulsion of parasites through the dog's feces.
Dosage Guidelines and Administration Instructions
Dosing is determined based on the dog's body weight:
- Small dogs (up to 25 lbs): One chewable per month
- Medium dogs (26-50 lbs): One chewable per month
- Large dogs (51-100 lbs): One chewable per month
Administer once monthly, year-round. It may be given with or without food. For best adherence, use digital calendars or mobile reminders. If a dose is missed, administer as soon as remembered, then resume the regular monthly schedule.

Storage, Shelf Life, and Handling Precautions
Store the product under the following conditions to maintain stability:
- Temperature: 15°C to 30°C (59°F to 86°F)
- Humidity: Store in a dry place
- Avoid exposure to sunlight, moisture, or excessive heat
Keep out of reach of children and non-target animals. Always refer to expiration labels, and dispose of unused product responsibly.
Side Effects and Tolerance in Dogs
Strongheart Plus is generally well-tolerated, but potential side effects include:
- Mild gastrointestinal disturbances such as vomiting, diarrhea, or flatulence
- Neurological effects including tremors or disorientation in ivermectin-sensitive breeds
- Hypersensitivity responses such as swelling, hives, or excessive scratching
Clinical experience suggests that adverse effects are infrequent and usually self-limiting.

Most Frequently Reported Common Side Effects
Veterinary data and owner observations indicate the following common reactions:
- Lethargy or decreased activity post-dose
- Increased drooling, nausea, or soft stools
- Occasional refusal to chew due to texture preference
- Temporary ataxia or imbalance in genetically susceptible breeds (e.g., Collies)

Contraindications and Breed Sensitivity
Do not use Strongheart Plus Chewable in the following situations:
- Puppies under 6 weeks of age or below the minimum weight threshold for labeled use
- Herding breeds (e.g., Collies, Shetland Sheepdogs) with MDR1 gene mutations that impair drug clearance
- Severely underweight or immunocompromised dogs due to altered drug metabolism
- Known hypersensitivity or allergy to ivermectin or pyrantel pamoate
Breed-specific testing may be warranted to avoid complications in sensitive canine populations.

Drug and Product Interactions
Strongheart Plus Chewable may interact with other veterinary pharmaceuticals, particularly those within the macrocyclic lactone class. Co-administration with additional antiparasitic agents such as milbemycin oxime or selamectin can potentiate neurological side effects, especially in genetically sensitive breeds.
Combining Strongheart Plus with certain sedatives or anesthetics may amplify central nervous system (CNS) effects, including depression or disorientation. This necessitates careful evaluation when managing dogs under long-term sedation or post-surgical analgesia.
Special caution is advised when used in conjunction with spinosad-based flea preventatives, as studies have shown increased risk of neurological reactions, particularly in Collies and related breeds.
To ensure safety and optimal therapeutic outcome, full disclosure of all ongoing medications, supplements, and preventatives to the attending veterinarian is imperative.

Special Precautions and Veterinary Warnings
Dogs with a history of seizure disorders require cautious administration. Ivermectin, a component of Strongheart Plus, may lower the seizure threshold in predisposed individuals.
Annual heartworm testing is essential before restarting therapy after any lapse in administration. Administering the chewable to an already infected dog may lead to severe reactions due to larval die-off.
Excessive or off-label use of antiparasitic agents can contribute to parasite resistance. Responsible rotation and use under veterinary guidance are crucial to maintain efficacy.
Chewables must be stored securely. Ingestion by children, cats, or other non-target species can result in serious adverse effects. Safe storage and post-use hygiene are non-negotiable safety practices.
Careful Administration in High-Risk or Compromised Dogs
For immunocompromised animals, whether due to disease, age, or concurrent medication, dosage and scheduling should be closely monitored and potentially modified based on individual response and veterinary discretion.
Dogs with hepatic or renal dysfunction may exhibit altered drug metabolism, potentially increasing systemic exposure. Periodic organ function tests may be warranted in these populations.
In cases involving gastrointestinal malabsorption syndromes, oral bioavailability may be compromised, necessitating alternative treatment approaches or dosage adjustments.
Use in Geriatric Dogs (Elderly Patients)
Senior dogs often exhibit decreased organ reserve, particularly in hepatic and renal systems. Routine monitoring of biochemical markers may guide safer administration.
An extended observation period following each dose is advisable to promptly identify any delayed or unusual reactions in elderly canines.
For geriatric dogs managing multiple comorbidities, polypharmacy considerations become critical. Each additional drug increases the potential for interaction and requires professional oversight.

Use in Pregnant, Breeding, or Lactating Dogs
Reproductive safety studies have shown no teratogenic effects from Strongheart Plus when used at recommended doses. It is generally considered safe during all stages of pregnancy and lactation.
Nonetheless, cautious use is advised during the first trimester unless clearly indicated. The decision should be based on a thorough risk-benefit analysis by the supervising veterinarian.
Studies confirm minimal drug transfer to breast milk. However, nursing puppies should be monitored for any abnormal signs to ensure tolerability during lactation.
Use in Puppies and Pediatric Dogs
Strongheart Plus is approved for use in puppies aged 6 weeks and older, provided they meet the minimum weight requirement established for each dosage strength.
Accurate weight-based dosing is critical to avoid underdosing, which can lead to therapeutic failure, or overdosing, which may result in toxicity.
Close observation during the first few doses is recommended, as immature systems may exhibit unique sensitivity to antiparasitic agents.
Overdose Symptoms and Emergency Treatment
Signs of ivermectin toxicity can include tremors, ataxia (loss of coordination), disorientation, hypersalivation, and in severe cases, seizures or coma. This is particularly concerning in MDR1-deficient breeds.
Emergency treatment protocols involve decontamination, such as induced vomiting and administration of activated charcoal, if ingestion occurred recently.
Supportive care may include IV fluids, anti-seizure medications, and hospitalization until the dog stabilizes. Recovery prognosis is favorable with prompt intervention and appropriate veterinary care.

Owner Handling and Safety Guidelines
Store Strongheart Plus Chewables in a secure, dry location, away from food, children's access, and other pets. Accidental ingestion by humans may cause dizziness, nausea, or more serious reactions in sensitive individuals.
If human exposure occurs, seek immediate medical advice and present the product label to the healthcare provider. Do not attempt to induce vomiting without professional guidance.
Always wash hands thoroughly after handling the chewable or coming into contact with the dog's mouth post-administration.
Unused or expired products should be disposed of following veterinary or local environmental disposal guidelines to avoid soil and water contamination.
Strongheart Plus Chewable FAQ
- What are the side effects of heartworm chewables?
- Is heartworm medication harmful to dogs?
- Are dogs healthy after heartworm treatment?
- How long can dogs live with heartworms?
- How long will a dog cough after heartworm treatment?
- How long does heartworm medicine stay in the dog's system?
- What are the signs of heartworm in dogs?
What are the side effects of heartworm chewables?
Depression/lethargy, vomiting, anorexia, diarrhea, mydriasis, ataxia, staggering, convulsions, and hypersalivation
Is heartworm medication harmful to dogs?
Ivermectin is generally considered safe for the majority of dogs. It has been proven to be highly efficient in both treating and preventing parasites.
Are dogs healthy after heartworm treatment?
If your dog is diagnosed with heartworm disease in its early stages and receives treatment promptly, it can lead to a good quality of life.
How long can dogs live with heartworms?
In such a state, dogs typically do not survive beyond a few weeks or months.
How long will a dog cough after heartworm treatment?
Few days or weeks
How long does heartworm medicine stay in the dog's system?
24 to 48 hours
What are the signs of heartworm in dogs?
Dogs with heartworm disease often exhibit a dry cough as one of the prominent symptoms, along with lethargic behavior and weight loss issues. They might also have a swollen belly and difficulty breathing as the disease progresses significantly.