Oncotaxel Injection
- Introduction to Oncotaxel Injection
- Composition and Pharmaceutical Form of Oncotaxel Injection
- Mechanism of Action: How Oncotaxel Injection Works
- Medical Uses of Oncotaxel Injection
- Dosage and Administration Guidelines for Oncotaxel Injection
- Docetaxel anhydrous side effects
- Drug Interactions with Oncotaxel Injection
- Docetaxel anhydrous warnings
- Contraindications for Oncotaxel Injection Use
- Careful Administration and Monitoring During Oncotaxel Therapy
- Special Considerations for Specific Populations
- Overdosage and Emergency Management of Oncotaxel
- Storage and Handling Instructions for Oncotaxel Injection
- Safe Handling Precautions for Healthcare Providers and Caregivers
Introduction to Oncotaxel Injection
Overview of Oncotaxel: Taxane-based Chemotherapy Agent
Oncotaxel Injection is a chemotherapy medication that includes docetaxel from the taxane group of drugs and is commonly used to treat various types of cancer. Its main function is as a cancer-fighting component that works by interfering with the microtubule network for cell division.
Brief History and Development
Originally derived from the European yew tree, taxanes have significantly advanced oncology treatments since the late 20th century. Docetaxel, a semi-synthetic derivative of paclitaxel, was developed to overcome certain limitations of early taxanes and received regulatory approval in the 1990s.

Approved Indications and Regulatory Status
Oncotaxel has received approval for treating types of cancer such as breast cancer, non-small cell lung cancer, prostate cancer, gastric adenocarcinoma, and head and neck cancers. It is authorized for use by health organizations, like the FDA and EMA.
Composition and Pharmaceutical Form of Oncotaxel Injection
Active Ingredient: Docetaxel Anhydrous
Each vial of Oncotaxel Injection contains docetaxel anhydrous in a carefully formulated solution designed to target and destroy cells effectively.
Inactive Ingredients and Excipients
- Polysorbate 80
- Dehydrated alcohol
- Citric acid and sodium citrate for pH stabilization
- Other buffering agents as needed for formulation integrity
Available Strengths and Presentation Forms
Oncotaxel is available in multiple strengths, typically 20 mg/mL and 80 mg/mL concentrations, packaged in single-dose vials for intravenous administration.
Mechanism of Action: How Oncotaxel Injection Works
Disruption of Microtubule Dynamics in Cancer Cells
Inhibition of Mitosis and Induction of Apoptosis
By inhibiting the breakdown of microtubules in cells, Oncotaxel halts the cell cycle at the G1 phase, leading to apoptosis and successful reduction of tumors.
Pharmacodynamics and Cell Cycle Specificity
Oncotaxel exhibits a high degree of cell cycle specificity, primarily targeting proliferative cells, making it a valuable asset against aggressive, rapidly dividing tumors.
Medical Uses of Oncotaxel Injection
FDA-Approved Uses
- Breast cancer: Early-stage and metastatic settings, often in combination regimens.
- Non-small cell lung cancer (NSCLC): As first-line or salvage therapy.
- Prostate cancer: Hormone-refractory metastatic disease.
- Gastric adenocarcinoma: Especially in advanced cases not amenable to surgery.
- Head and neck cancers: Including locally advanced squamous cell carcinomas.
Off-Label Uses of Oncotaxel Injection
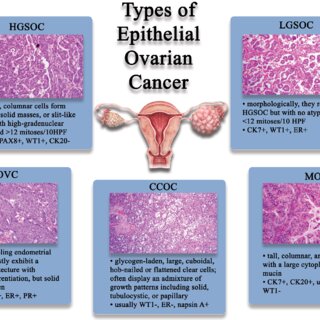
- Ovarian cancer
- Bladder cancer
- Esophageal cancer
- Soft tissue sarcomas
- Pancreatic cancer
- Endometrial carcinoma
Dosage and Administration Guidelines for Oncotaxel Injection
Standard Dosing Schedules by Indication
Doses vary from 60 mg/m² to 100 mg/m², administered via intravenous infusion over one hour every three weeks, depending on cancer type and patient condition.
Dose Modifications Based on Hematologic and Non-Hematologic Toxicities
Dosage adjustments are critical in patients experiencing significant neutropenia, hepatotoxicity, or severe mucositis.
Premedication with Corticosteroids to Prevent Hypersensitivity and Fluid Retention
Patients are often given corticosteroids, like dexamethasone, before procedures to reduce the chances of reactions and capillary leakage syndrome.
Administration Technique: Intravenous Infusion Protocol
Oncotaxel must be administered using in-line filters, through a controlled infusion over 1 hour, ensuring stable plasma concentrations.
Duration and Frequency of Treatment Cycles
Cycles are repeated every 21 days, with treatment durations tailored according to tumor response and patient tolerability.
Docetaxel anhydrous side effects
Common Side Effects
- Neutropenia and febrile neutropenia: Frequent and dose-limiting toxicities requiring close monitoring.
- Alopecia: Almost universal in treated patients, reversible upon therapy cessation.
- Fatigue and weakness: Often cumulative over successive cycles.
- Nausea, vomiting, and diarrhea: Manageable with prophylactic antiemetics and supportive care.
- Nail changes: Including onycholysis, subungual hemorrhage, and discoloration.

Serious Adverse Effects
- Severe hypersensitivity reactions: Manifesting as bronchospasm, hypotension, and urticaria.
- Cardiotoxicity and arrhythmias: Rare but serious, particularly in pretreated individuals.
- Pulmonary toxicity and interstitial lung disease: Requiring early detection and management.
- Peripheral neuropathy: Dose-dependent, often necessitating therapy modification.
- Hepatic impairment and hepatotoxicity: Routine liver function monitoring advised.

Drug Interactions with Oncotaxel Injection
Interaction with CYP3A4 Inhibitors and Inducers
Strong CYP3A4 inhibitors (e.g., ketoconazole, ritonavir) can elevate docetaxel plasma levels, while inducers (e.g., rifampin) may decrease efficacy.
Potential Interactions with Anticoagulants and Anti-infectives
Concomitant use with warfarin or antifungal agents requires vigilant INR monitoring and infection prophylaxis strategies.
Risk of Additive Toxicity with Other Chemotherapeutic Agents
Combination with other cytotoxics heightens risks of myelosuppression and necessitates dose adjustments and intensified monitoring.
Docetaxel anhydrous warnings
Risk of Severe Hypersensitivity Reactions and Anaphylaxis
Premedication and readiness for emergency management are mandatory before initiating therapy with Oncotaxel.
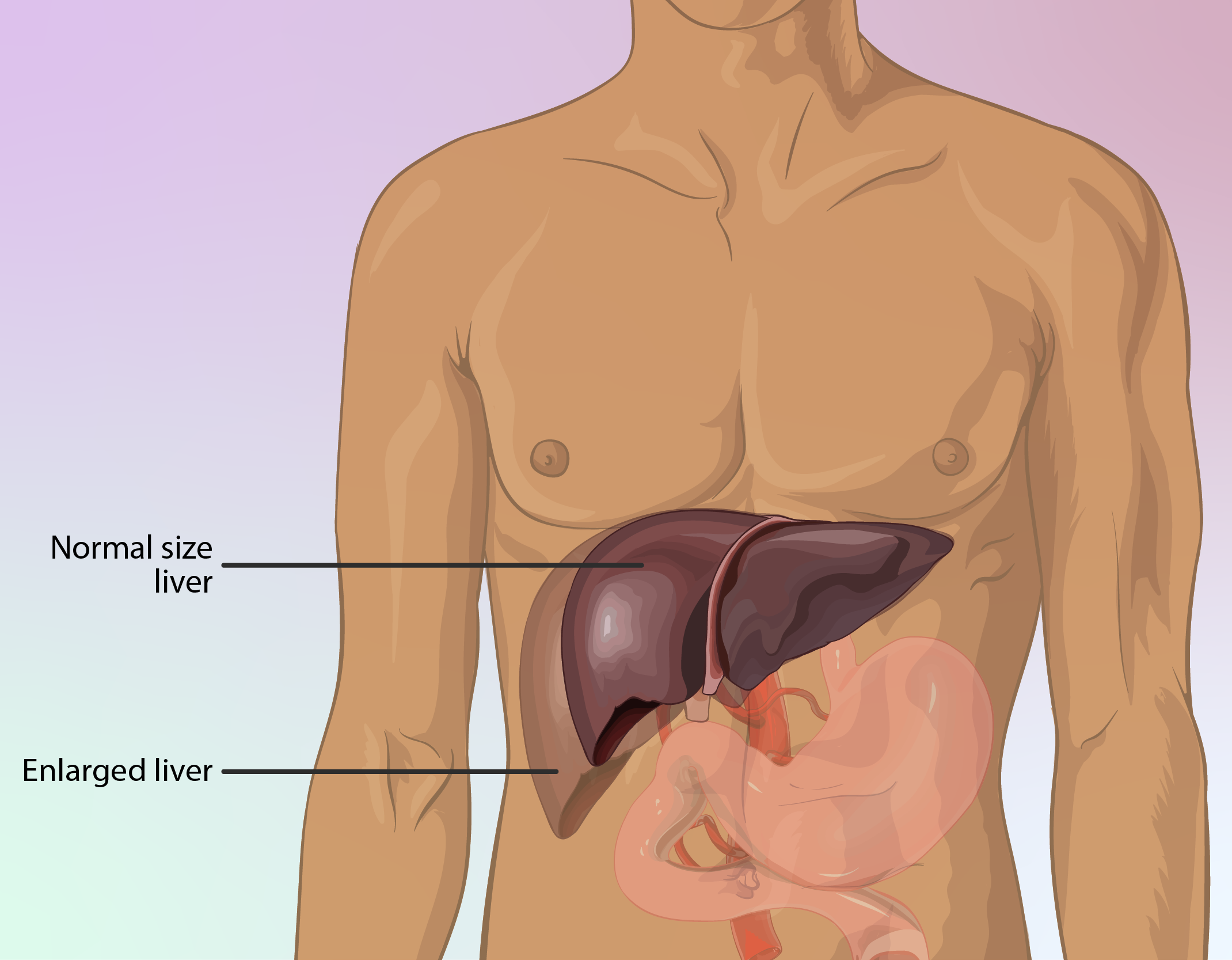
Need for Liver Function Monitoring Before and During Treatment
Liver problems could significantly raise the risk of effects, so treatment may need to be paused or modified for patients with liver enzyme issues.
Bone Marrow Suppression and Infection Risk
Profound neutropenia exposes patients to a high risk of serious infections, warranting the use of prophylactic measures such as granulocyte colony-stimulating factors (G-CSFs) when appropriate.
Fluid Retention and Edema Management
Peripheral edema and pleural effusions may necessitate diuretics, dose modifications, or discontinuation depending on severity.
Contraindications for Oncotaxel Injection Use
Known Hypersensitivity to Docetaxel or Polysorbate 80
Patients who have shown reactions to docetaxel or polysorbate 80 should avoid using Oncotaxel Injection due to the risk of adverse reactions, such as bronchospasm or anaphylaxis.
Baseline Neutrophil Counts Below Threshold Levels
Patients presenting with a baseline neutrophil count below 1,500 cells/mm³ should not receive Oncotaxel. Treatment under these conditions significantly heightens the risk of life-threatening infections and sepsis.
Severe Hepatic Impairment
Severe hepatic dysfunction markedly increases the systemic exposure to docetaxel, thereby intensifying toxicity. Oncotaxel is contraindicated in cases of elevated transaminases exceeding 1.5 times the upper limit of normal (ULN) in conjunction with elevated alkaline phosphatase.
History of Severe Hypersensitivity to Other Taxanes
Cross-reactivity has been observed among taxanes, including paclitaxel and docetaxel. A documented history of severe hypersensitivity to any taxane class agent precludes the safe use of Oncotaxel Injection.
Careful Administration and Monitoring During Oncotaxel Therapy
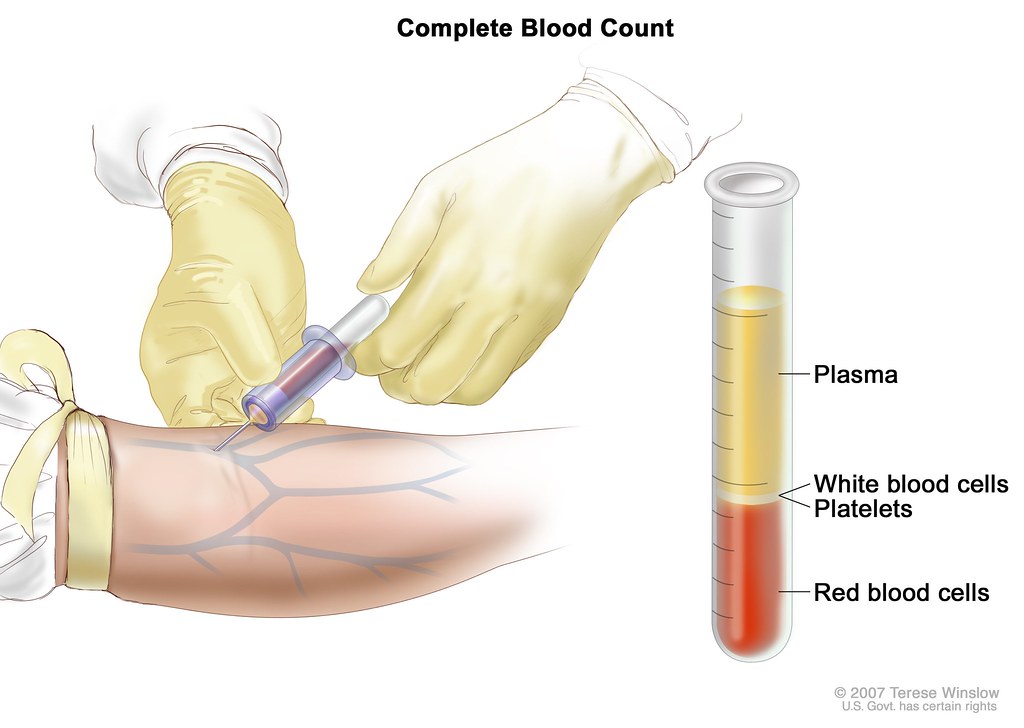
Regular Complete Blood Count (CBC) Monitoring
Frequent hematologic monitoring, particularly of white blood cell and neutrophil counts, is paramount. CBCs are typically obtained prior to each treatment cycle and at nadir points to preemptively identify myelosuppression.
Liver Function Test (LFT) Assessments
Periodic liver function evaluations are essential due to the hepatic metabolism of docetaxel. Transaminases, bilirubin, and alkaline phosphatase levels must be assessed to guide dosing decisions and avoid cumulative toxicity.
Cardiac Monitoring in Patients with Preexisting Heart Conditions
Patients with underlying cardiac disorders should undergo baseline and periodic cardiac evaluations, including echocardiograms or ECGs. This vigilance helps detect subclinical declines in cardiac function, especially in those with previous anthracycline exposure.
Pulmonary Evaluation in Case of Respiratory Symptoms
Emergent respiratory complaints warrant immediate pulmonary investigations. Imaging studies, including chest radiographs or CT scans, may reveal interstitial lung changes necessitating treatment discontinuation.
Special Considerations for Specific Populations
Administration to Elderly Patients
Dose Adjustment Considerations
As people get older, they may need doses of medication because their organs may not work well due to aging processes. They might start with lower doses and need to be extra cautious for potential side effects on the blood and other parts of the body.
Increased Risk of Toxicities and Supportive Care Strategies
Elderly patients face amplified risks of neutropenic complications, mucositis, and neuropathy. Prophylactic interventions such as growth factor support and aggressive symptom management enhance therapy tolerability.
Administration to Pregnant Women and Nursing Mothers
Category D: Known Fetal Risk Based on Human Data
Oncotaxel presents risks of birth defects and harm to embryos. Avoid using it during pregnancy as it is classified as Category D for pregnancy; the benefits must clearly outweigh the risks to the fetus.
Recommendation to Avoid Use During Pregnancy
Women of childbearing age are recommended to use birth control methods while undergoing treatment and continue to do for a few months after the treatment ends to avoid any unplanned pregnancies, during this period.
Contraindicated During Breastfeeding
Owing to the potential for serious adverse reactions in nursing infants, breastfeeding must be discontinued prior to commencing Oncotaxel therapy and should remain contraindicated throughout treatment duration.

Administration to Pediatric Patients
Limited Safety and Efficacy Data
Clinical evidence regarding Oncotaxel use in pediatric populations remains sparse. As such, its safety, efficacy, and dosing parameters in children are not well-established, necessitating cautious, case-by-case evaluation.
Special Dosing Considerations in Clinical Trials and Off-Label Settings
When employed off-label or in experimental protocols, pediatric dosing strategies often adjust adult dosages based on body surface area, with careful toxicity monitoring to minimize adverse outcomes.
Overdosage and Emergency Management of Oncotaxel
Symptoms of Acute Overdose: Severe Neutropenia, Mucositis, Sensory Neuropathy
Acute overdose of Oncotaxel may precipitate profound neutropenia, severe mucosal inflammation, and peripheral neuropathies. Without swift intervention, these sequelae can escalate to life-threatening complications.
Immediate Supportive Treatment Protocols
Management places emphasis on providing treatment that involves giving growth factors and broad-spectrum antibiotics, along with administering intravenous fluids to ensure hydration levels are maintained appropriately; in addition to this approach, it includes treating mucositis and neuropathy symptoms as needed.
No Specific Antidote; Emphasis on Symptomatic Management
No direct antidote exists for docetaxel toxicity. Therefore, therapy is centered on vigilant clinical support and mitigation of secondary complications until hematologic and mucosal recovery occurs.
Storage and Handling Instructions for Oncotaxel Injection
Recommended Storage Temperatures and Conditions
Oncotaxel vials need to be kept in the refrigerator at temperatures between 2 °C and 8°C (36°F and 46°F). It's important to avoid freezing them in order to maintain the integrity of the formulation.
Protection from Light and Freezing
Vials must be maintained in original cartons to shield from light exposure. Both undiluted and diluted solutions are sensitive to light and should be protected during storage and infusion.
Shelf-life and Reconstitution Guidelines for Prepared Solutions
- Unopened vials remain stable until the expiration date printed on the packaging.
- Once diluted for infusion, solutions are typically stable for up to 4 hours at room temperature if protected from light.
- Strict aseptic preparation is mandatory to minimize contamination risks.
Safe Handling Precautions for Healthcare Providers and Caregivers
Use of Personal Protective Equipment (PPE) During Preparation and Administration
Healthcare personnel must utilize PPE, including gloves, gowns, and face protection, when handling Oncotaxel to prevent dermal and mucosal exposure to the cytotoxic agent.
Guidelines for Spill Management and Accidental Exposure
Spill kits designed for cytotoxic drug containment should be readily accessible. In case of accidental skin contact, the affected area must be washed immediately with copious water and medical evaluation sought if necessary.
Proper Disposal Procedures for Unused Product and Contaminated Materials
Please ensure that any Oncotaxel solution and contaminated protective equipment (PPE), as well as administration materials, should be properly disposed of following the local regulations related to hazardous pharmaceutical waste management.
Oncotaxel Injection FAQ
- What kind of chemo is paclitaxel?
- What is the most concerning side effect of paclitaxel?
- What is the action of paclitaxel?
- How successful is paclitaxel?
- How long is paclitaxel treatment?
- What not to eat with paclitaxel?
- Can paclitaxel shrink tumors?
- What is the life expectancy of someone on paclitaxel?
- Do you lose your hair with paclitaxel?
- What is the survival rate for paclitaxel?
- Why is paclitaxel given weekly?
- When should I stop paclitaxel?
- What are the side effects of paclitaxel?
- Is paclitaxel safe?
- How long does it take to recover from paclitaxel?
- Is paclitaxel toxic to the liver?
- How long can you stay on paclitaxel?
- What kind of chemo is paclitaxel?
- What is the action of paclitaxel?
- How long is paclitaxel treatment?
- Do you lose your hair with paclitaxel?
- What is the survival rate for paclitaxel?
- Why is paclitaxel given weekly?
- Is paclitaxel safe?
What kind of chemo is paclitaxel?
Paclitaxel is classified as an antineoplastic medication that works by disrupting the growth of cancer cells, leading to their elimination.
What is the most concerning side effect of paclitaxel?
Nausea and vomiting that persists without relief; indications of anemia, like fatigue and paleness in the skin; frequent bruising or bleeding with minimal injury; sudden loss of consciousness; cognitive disorientation; discomfort or inflammation accompanied by weakness in the arms or legs; warmth and swelling, in the calves; expelling coughed up matter
What is the action of paclitaxel?
Paclitaxel works by stabilizing microtubules and interfering with the formation of the spindle in cancer cells to slow down their growth rate effectively.
How successful is paclitaxel?
50% of patients
How long is paclitaxel treatment?
A typical cycle usually spans around three weeks, which is equivalent to 21 days. However, there are instances where it extends to four weeks totaling 28 days.
What not to eat with paclitaxel?
Grapefruits and grapefruit juice
Can paclitaxel shrink tumors?
Yes
What is the life expectancy of someone on paclitaxel?
11.9 months
Do you lose your hair with paclitaxel?
Yes
What is the survival rate for paclitaxel?
39% 2-year survival rate
Why is paclitaxel given weekly?
Administering doses frequently could be more effective than the typical dosage every three weeks, since it allows for continuous exposure of actively dividing cancer cells to its harmful effects.
When should I stop paclitaxel?
After 4 cycles
What are the side effects of paclitaxel?
Side effects may include the risk of infection, decreased blood cell count, anemia, diarrhea, mouth and throat soreness, muscle or joint discomfort, fluctuations in blood pressure, skin alterations, and hand-foot syndrome (palmar–plantar syndrome).
Is paclitaxel safe?
Paclitaxel has the potential to lead to scarring or inflammation in the lung tissue.
How long does it take to recover from paclitaxel?
2 months
Is paclitaxel toxic to the liver?
Mildly impaired liver function
How long can you stay on paclitaxel?
Up to 8 cycles, which can take up to 6 months
What kind of chemo is paclitaxel?
Paclitaxel is classified as a drug that inhibits the proliferation of cancer cells, leading to their eradication.
What is the action of paclitaxel?
Paclitaxel works by stabilizing microtubules and interfering with the formation of the cell division spindle in a way that's harmful to cancer cells, inhibiting their rapid growth.
How long is paclitaxel treatment?
Typically, a cycle spans about 3 weeks, which is equivalent to 21 days, though occasionally it can extend to 4 weeks, totaling 28 days.
Do you lose your hair with paclitaxel?
Yes
What is the survival rate for paclitaxel?
39% 2-year survival rate
Why is paclitaxel given weekly?
Administering doses frequently could be more effective than the standard three-week dosing schedule since it allows for prolonged exposure of dividing tumor cells to the treatment's cytotoxic effects.
Is paclitaxel safe?
Paclitaxel may lead to scarring or inflammation in the lung tissue.