1. Introduction to Tastylia (Tadalafil Oral Strip)
1.1 Overview of Tastylia and its Unique Oral Strip Formulation
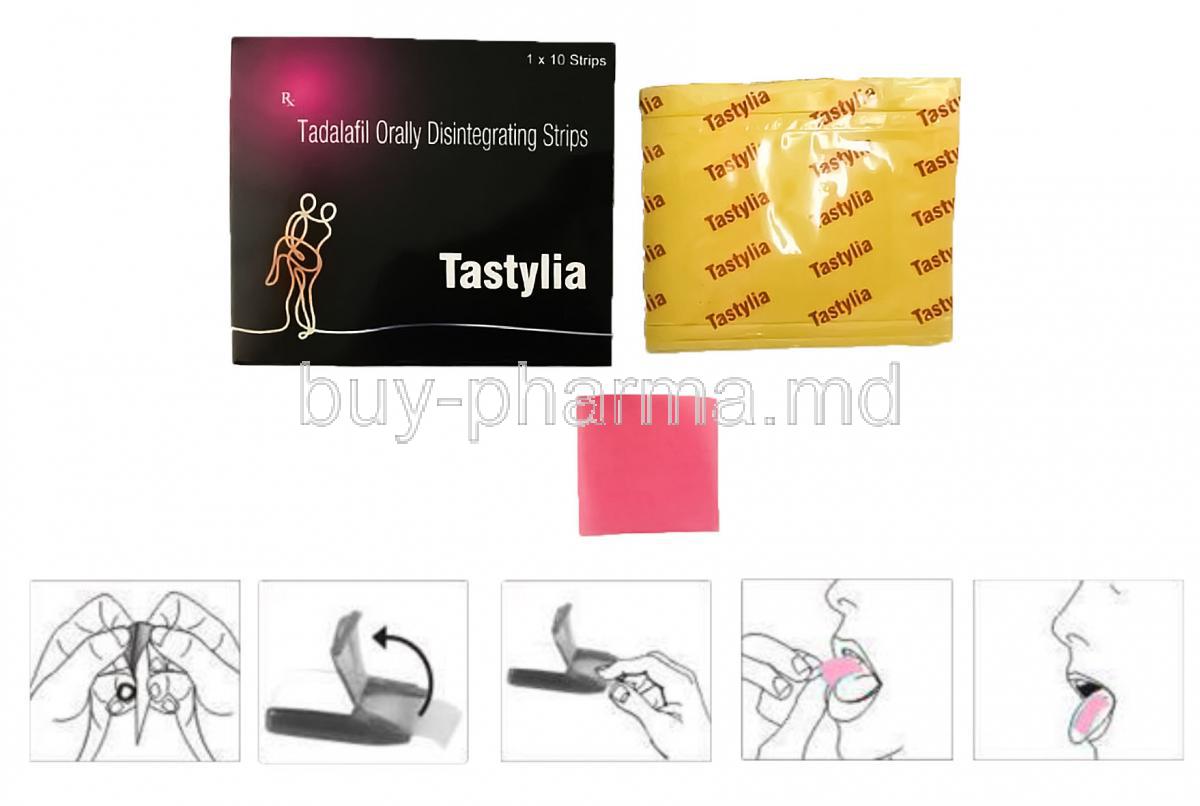
Tastylia is a novel erectile dysfunction (ED) therapy that delivers tadalafil through an orally disintegrating strip. Designed for rapid sublingual absorption, it bypasses gastrointestinal degradation and hepatic first-pass metabolism, allowing for faster onset of action. Its discreet, easy-to-carry form provides a convenient alternative to traditional tablets.
1.2 Comparison with Traditional Tadalafil Tablets and Other ED Medications
Unlike conventional tablets such as Cialis or generic tadalafil, Tastylia does not require water for administration. Its oral strip form ensures direct mucosal absorption, which significantly reduces latency time. Compared to sildenafil (Viagra), Tastylia’s effects last longer, up to 36 hours, providing greater spontaneity and flexibility.
1.3 Key Benefits of Sublingual/Oral Strip Delivery for Faster Absorption
- Rapid onset within 15–30 minutes
- No need for water or swallowing
- Ideal for patients with dysphagia or pill aversion
- More consistent plasma concentration due to mucosal uptake
1.4 Regulatory Status and Availability in Different Markets
Tastylia is marketed in select international markets as a nutraceutical or prescription ED treatment, depending on local regulatory frameworks. It is available in various strengths and often classified under wellness or functional sexual health products in over-the-counter sectors.
2. Composition and Active Ingredients
2.1 Tadalafil as the Primary Active Ingredient
Each Tastylia oral strip contains tadalafil, a potent phosphodiesterase type 5 (PDE5) inhibitor that facilitates erections by relaxing penile smooth muscle and enhancing blood flow.
2.2 Available Strengths and Dosage Options
Tastylia typically comes in 10 mg and 20 mg doses, though some formulations may include lower (5 mg) or higher strengths (40 mg) for off-label or daily use protocols.
2.3 Inactive Components and Excipients in Oral Strip Formulation
- Film-forming polymers
- Plasticizers and sweeteners (e.g., sucralose)
- Flavoring agents for palatability
- Stabilizers and preservatives
2.4 Pharmaceutical Technology Behind Rapid-Dissolving Oral Strips
The strip is manufactured using solvent casting and drying techniques, yielding a thin, flexible film that dissolves upon contact with saliva. This ensures near-immediate release of the active ingredient into the oral mucosa.
3. Mechanism of Action: How Tastylia Works
3.1 PDE5 Inhibition and the Role of Tadalafil in Erectile Function
Tadalafil inhibits the PDE5 enzyme, which breaks down cyclic guanosine monophosphate (cGMP). This prolongs vasodilation in the corpus cavernosum, essential for sustained erections.
3.2 Enhancement of Penile Blood Flow and Nitric Oxide Pathway
Nitric oxide (NO) release during arousal stimulates guanylate cyclase, increasing cGMP. Tadalafil augments this effect, leading to enhanced arterial inflow and restricted venous outflow, promoting erection.
3.3 Onset of Action and Duration Compared with Tablets
Tastylia strips typically act within 15–30 minutes and remain effective for up to 36 hours. This onset is faster than conventional tablets, whose absorption is delayed by gastric emptying or food intake.
3.4 Physiological Response During Sexual Stimulation
Tadalafil requires sexual arousal to activate its pharmacologic action. It does not induce spontaneous erections; instead, it enhances the natural erectile response through smooth muscle relaxation.
4. Approved Medical Uses of Tastylia
4.1 Erectile Dysfunction (ED) Treatment and Sexual Performance Enhancement
Primarily indicated for the treatment of ED, Tastylia helps men achieve and maintain erections sufficient for satisfactory sexual activity. It is effective across a wide age spectrum and in men with comorbidities like diabetes or hypertension.
4.2 Benign Prostatic Hyperplasia (BPH) Symptom Relief
Tadalafil’s smooth muscle relaxation also impacts the prostate and bladder neck, relieving urinary symptoms associated with BPH such as weak stream and urgency.
4.3 Combined ED and BPH Therapy in Men
For men suffering from both ED and BPH, Tastylia provides a dual therapeutic benefit with a single agent, improving compliance and quality of life.
4.4 Pulmonary Arterial Hypertension (PAH) in Adult Patients
Although oral strips are not the standard form for PAH treatment, tadalafil itself is FDA-approved for this condition due to its vasodilatory effects on pulmonary arteries, reducing pulmonary pressure and enhancing exercise capacity.
5. Off-Label and Investigational Uses
5.1 Daily Low-Dose Therapy for Long-Term ED Management
Off-label use of low-dose tadalafil (2.5–5 mg) daily has shown benefit in maintaining erectile function and spontaneity, especially in men who prefer continuous coverage rather than on-demand treatment.
5.2 Use in Penile Rehabilitation Post-Prostate Surgery
Tadalafil has been explored for post-operative recovery of erectile function in men undergoing radical prostatectomy, helping restore nocturnal erections and tissue oxygenation.
5.3 Sports and Athletic Endurance Enhancement (Not FDA-Approved)
There is anecdotal and investigational interest in tadalafil’s ability to improve oxygenation and muscular blood flow, although its use in sports remains controversial and is banned by some athletic authorities.
5.4 Potential Vascular and Circulatory Health Benefits Under Research
Emerging data suggest tadalafil may exert beneficial effects on endothelial function, systemic blood pressure, and even lower limb circulation, though such uses remain experimental.
6. Dosage and Administration Guidelines
6.1 Standard Single-Use Dosing for ED Treatment
A typical single dose is 10 mg or 20 mg taken at least 30 minutes before anticipated sexual activity. It should not be used more than once per day.
6.2 Recommended Timing and Frequency for Optimal Effect
- Take on an empty stomach for faster effect
- Do not exceed one strip in 24 hours
- Avoid simultaneous use with other ED medications
6.3 Daily Low-Dose Regimen Versus On-Demand Usage
Tastylia may be used in daily low-dose regimens under physician supervision. This approach is suitable for men with chronic ED or combined ED and BPH.
6.4 Sublingual/Oral Strip Administration Instructions for Maximum Absorption
- Place strip under the tongue and allow it to dissolve completely
- Do not chew or swallow immediately
- Avoid eating or drinking until the strip has fully dissolved
6.5 Dose Adjustments for Patients with Liver or Kidney Impairment
Patients with hepatic or renal dysfunction may require lower starting doses. In moderate-to-severe impairment, use is discouraged or contraindicated without medical guidance.
7. Side Effects of Tastylia
7.1 Overview of Mild to Severe Side Effects
Tastylia is generally well-tolerated, but like all PDE5 inhibitors, it can produce adverse effects ranging from mild discomfort to serious medical events.
7.2 Common Side Effects: Headache, Flushing, Nasal Congestion, Indigestion
- Headache and facial flushing due to vasodilation
- Stuffy or runny nose from mucosal congestion
- Dyspepsia and acid reflux-like symptoms
7.3 Less Common Adverse Reactions: Back Pain, Dizziness, Muscle Aches
These effects are usually transient and resolve spontaneously. Hydration and rest can alleviate symptoms in most cases.
7.4 Serious Side Effects: Vision or Hearing Loss, Priapism, Cardiovascular Complications
Sudden visual or auditory impairment, painful erections lasting over 4 hours (priapism), and cardiovascular events such as chest pain or arrhythmia require immediate cessation and emergency care.
7.5 When to Seek Medical Attention Immediately
Any signs of allergic reaction (rash, swelling, breathing difficulty), chest pain, or prolonged erection should prompt urgent medical evaluation.
8. Drug Interactions and Contraindications
8.1 Interaction with Nitrates and Nitric Oxide Donors
Concurrent use with nitrates (e.g., nitroglycerin) can result in life-threatening hypotension and is strictly contraindicated.
8.2 Interactions with Alpha-Blockers and Antihypertensive Drugs
Caution is advised when used with blood pressure medications. Start at the lowest dose to avoid additive hypotensive effects.
8.3 CYP3A4 Inhibitors and Inducers Affecting Tadalafil Metabolism
- Inhibitors (e.g., ketoconazole, ritonavir) increase tadalafil levels
- Inducers (e.g., rifampin, phenytoin) reduce efficacy
8.4 Contraindicated Conditions: Severe Heart Disease, Low Blood Pressure, Recent Stroke/Heart Attack
Patients with unstable angina, recent myocardial infarction, or hypotension should avoid PDE5 inhibitors. Tadalafil may exacerbate cardiac risk.
8.5 Contraindication in Hypersensitivity to Tadalafil or Excipients
Any history of allergic reactions to tadalafil or components in the oral strip necessitates avoidance and alternative therapy selection.
9. Warnings and Important Precautions
9.1 Cardiovascular Risk Assessment Before Use
A thorough cardiovascular evaluation is recommended, especially in men over 50 or with comorbidities. ED can be an early indicator of heart disease.
9.2 Avoiding Combination with Other PDE5 Inhibitors or ED Medications
Using multiple PDE5 inhibitors concurrently increases the risk of hypotension, priapism, and other side effects. Only one ED drug should be used at a time.
9.3 Alcohol and Grapefruit Interactions Affecting Efficacy and Safety
Alcohol can impair erectile performance and increase vasodilation-related side effects. Grapefruit may elevate tadalafil concentrations through CYP3A4 inhibition.
9.4 Risk of Priapism and How to Prevent Prolonged Erections
Patients should be educated to seek help if an erection lasts longer than four hours. Avoiding excessive doses and combination with other ED drugs minimizes this risk.
9.5 Visual and Auditory Disturbances: Rare but Critical Warnings
Though rare, non-arteritic anterior ischemic optic neuropathy (NAION) and sudden hearing loss have been reported. Discontinue use and consult an ophthalmologist or ENT specialist if symptoms arise.
10. Careful Administration in Special Populations
10.1 Administration to Elderly Patients
Elderly individuals may exhibit increased pharmacodynamic sensitivity to tadalafil. This heightened response is primarily due to age-related physiological changes, including reduced hepatic metabolism and renal clearance, which can amplify the drug’s systemic effects.
- Start with the lowest effective dose to minimize adverse reactions.
- Close monitoring of blood pressure and heart rate is recommended due to potential cardiovascular strain.
- Periodic evaluation of hepatic and renal function is advisable during long-term use.
Given the increased prevalence of comorbidities in this demographic—such as coronary artery disease, hypertension, or diabetes—thorough assessment before initiating therapy is essential.
10.2 Use in Pregnant Women and Nursing Mothers
Tadalafil is not intended for use in women; however, incidental exposure may occur. Animal studies have shown no teratogenic effects at clinically relevant doses, but adequate human studies are lacking.
- Use during pregnancy should be avoided unless clearly necessary and prescribed under physician supervision.
- It is unknown whether tadalafil is excreted in human breast milk. However, due to the potential for adverse effects in nursing infants, discontinuation of either the drug or breastfeeding may be required.
Given the absence of therapeutic indications in pregnant or lactating women, caution is emphasized, and exposure should generally be avoided.
10.3 Administration to Children and Adolescents
Tadalafil has not been studied adequately in pediatric populations. Safety and efficacy data in individuals under 18 years of age are nonexistent, and as such, its use is contraindicated.
- No pediatric-approved indication currently exists for Tastylia or other tadalafil formulations.
- Potential hormonal or vascular effects in developing bodies pose theoretical risks.
Under no circumstances should Tastylia be administered to children or adolescents, even in cases of off-label curiosity.
11. Overdose and Emergency Management
11.1 Symptoms of Tadalafil Overdose and Potential Complications
Overconsumption of tadalafil, whether accidental or intentional, may lead to a range of toxic manifestations. These typically reflect exaggerated pharmacological effects.
- Severe hypotension and fainting
- Persistent and painful erections (priapism)
- Blurred vision, photophobia, or sudden vision loss
- Chest pain, palpitations, or cardiac arrhythmias
In rare cases, renal dysfunction or seizures may occur due to secondary hemodynamic collapse.
11.2 Immediate Steps and Emergency Medical Interventions
Upon suspected overdose, the individual should receive urgent medical evaluation. There is no specific antidote for tadalafil overdose; management is symptomatic and supportive.
- Stabilize blood pressure using IV fluids and vasopressors if necessary.
- Administer oxygen or beta-blockers in cases of cardiac symptoms.
- In cases of priapism, intracavernosal injection of sympathomimetic agents or surgical intervention may be required.
11.3 Monitoring Cardiovascular and Renal Function in Overdose Cases
Continuous cardiac monitoring is vital in patients with cardiovascular compromise. Renal function should be assessed and managed in the event of fluid shifts, dehydration, or pre-existing nephropathy.
Recovery is usually complete with appropriate intervention, but observation should continue for a minimum of 24 hours in moderate to severe overdose scenarios.
12. Storage and Handling Precautions
12.1 Recommended Storage Temperature and Humidity Conditions
Tastylia oral strips should be stored at controlled room temperature, ideally between 20°C and 25°C (68°F–77°F). Fluctuations outside this range can affect the drug’s stability and efficacy.
- Avoid refrigeration or exposure to heat sources.
- Humidity should be kept below 60% to prevent strip degradation.
12.2 Protecting Oral Strips from Light and Moisture Exposure
Each strip is sensitive to light and moisture, which may compromise its integrity and bioavailability. Always store in the original sealed packaging until ready for use.
- Do not open the sachet prematurely.
- Keep away from sinks, bathrooms, or steamy environments.
12.3 Safe Handling to Maintain Strip Integrity
The oral strip is delicate and should be handled with dry, clean hands. Avoid bending, tearing, or crushing the strip before administration.
Improper handling may result in dose loss, delayed dissolution, or uneven absorption.
12.4 Shelf Life and Disposal of Unused or Expired Strips
Check the expiry date on each sachet prior to use. Expired strips should not be consumed as chemical degradation may reduce potency and safety.
- Do not flush unused strips into the toilet.
- Dispose of in accordance with local pharmaceutical waste guidelines.
Safe disposal minimizes environmental contamination and prevents accidental ingestion by children or pets.