Zolephos Infusion, Zoledronate
- Introduction
- Overview of Zolephos Infusion (Zoledronate)
- Composition and Formulation
- Aclasta vs Zoledronate
- Zoledronate vs Zoledronic
- Zoledronate vs Reclast
- Zoledronate vs Ibandronate
- Zoledronate vs Risedronate
- Zoledronate vs Denosumab
- Zoledronate vs Zoledronic acid
- Zoledronate vs Pamidronate
- Zoledronate vs Prolia
- Zoledronate vs Alendronate
- Mechanism of Action: How Zolephos Infusion Works
- Approved Medical Uses
- Treatment of osteoporosis in postmenopausal women and men
- Prevention of fractures in patients with osteoporosis
- Treatment and prevention of glucocorticoid-induced osteoporosis
- Treatment of Pagets disease of bone
- Management of bone metastases from solid tumors
- Reduction of skeletal-related events in multiple myeloma
- Zoledronate for hypercalcemia
- Zoledronate for breast cancer
- Zoledronate for osteosarcoma in dogs
- Zoledronate for horses
- Zoledronate for osteogenesis imperfecta
- Zoledronate for osteosarcoma
- Zoledronate for metastatic breast cancer
- Zoledronate for osteopenia
- Zoledronate for myeloma
- Off-Label Uses
- Dosage and Administration
- Special Populations and Administration Guidance
- Warnings and Precautions
- Contraindications
- Careful Administration and Important Precautions
- Drug Interactions
- Side Effects
- Common Side Effects
- Overdose and Emergency Management
- Handling and Storage Precautions
Introduction
The Zolephos infusion, which contains zoledronic acid (a potent bisphosphonate), is an essential tool in managing various skeletal disorders. It is highly effective in inhibiting bone erosion, thereby maintaining bone integrity in patients with malignancy-related bone disease and metabolic conditions. Clinically, particularly in oncology, this treatment represents a vital advance in preserving patient quality of life, making it a subject of considerable scientific and clinical interest across oncology, endocrinology, and orthopedics.
Overview of Zolephos Infusion (Zoledronate)
Zolephos Infusion is an IV formulation of acid that targets the osteoclasts responsible, for bone breakdown, thereby dampening bone resorption. By curbing this activity, it helps keep problems—such as fractures, spinal‑cord compression, and the hypercalcemia that can accompany malignancy—under control. Administered in hospitals or dedicated outpatient clinics the infusion delivers the drug in a manner producing pharmacokinetics that are both predictable and consistent.
Historical development and approval status
Developed in the late 20th century, zoledronic acid emerged as a more refined form of bisphosphonate therapy. After successful clinical trials, it gained regulatory approval in the early 2000s for treating bone metastases, multiple myeloma, and osteoporosis. Over time, its clinical applications have expanded due to its demonstrated versatility and favorable safety profile across global markets.
Importance in oncology and bone health management
In oncology, Zolephos Infusion trims down the skeletal‑related events that curtail mobility and chip away at patients’ independence. For those dealing with osteoporosis or metabolic bone disease, it provides fracture protection. This infusion isn’t another option; it often becomes a pivotal factor shaping long‑term outcomes. Its importance can be summed up as follows:
It reduces the risk of bone fractures in cancer patients whose disease has taken hold of their bones. Effectively reins in cancer‑driven calcium surges. It works to boost bone density while managing osteoporosis. Fosters a quality of life by preserving the skeletal architecture.
Composition and Formulation
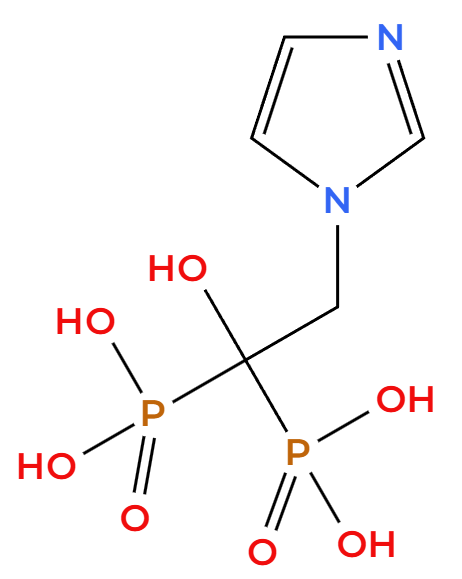
Active ingredient: Zoledronic acid (Zoledronate)
The core component, zoledronic acid, is a highly effective nitrogen-containing bisphosphonate. It works by attaching directly to the hydroxyapatite crystals within the bone, which powerfully restricts osteoclast activity and thus curtails bone resorption (breakdown). This molecular design ensures the drug has a strong affinity for bone tissue, giving it a long-lasting effect.
Excipients and stabilizers
The usual mix is built around water for injection with mannitol playing the role of a stabilizer and sodium citrate serving as a buffering agent. This trio of excipients works to keep the preparation soluble and potent both, during storage and, at the point of administration.
Available strengths and packaging forms
Typically Zolephos Infusion comes in vials each delivering a 4 mg solution of acid. While the exact packaging may differ among manufacturers the product consistently adheres to strict sterility and stability standards.
Aclasta vs Zoledronate
Aclasta is the trade name for zoledronic acid, primarily prescribed for osteoporosis and Paget’s disease. Its generic counterpart is called zoledronate. Though the two are chemically identical the brand label often signals differences in approved indications and the way the product is marketed.
Zoledronate vs Zoledronic
These labels are habitually swapped in conversation. "Zoledronate" identifies the salt variant, whereas "acid" points to the parent molecule. Yet both ultimately converge on the active chemical.
Zoledronate vs Reclast
Reclast is the brand name used for zoledronic acid when it is prescribed for osteoporosis treatment. The fundamental difference between this and other zoledronic acid products is not the chemical compound itself, but rather the regulatory classification and the specific marketing brand used.
Zoledronate vs Ibandronate
While ibandronate is typically administered orally or via intravenous (IV) injection, zoledronate is significantly more potent and is usually given as a once-yearly infusion. This higher potency makes zoledronate the preferred option for patients needing a powerful effect on bone turnover. Ultimately, the choice between the two drugs is determined by the patient's risk of fracture and their ability to adhere to the treatment schedule.
-injection.jpg)
Zoledronate vs Risedronate
Risedronate is a weekly or monthly oral medication requiring strict adherence to prevent stomach irritation. Conversely, Zoledronate is delivered intravenously, bypassing the digestive system entirely, which often provides more reliable outcomes for patients at high risk.
Zoledronate vs Denosumab
Denosumab, a monoclonal antibody, acts by blocking the RANKL pathway to prevent new bone-dissolving cells from forming, and it is administered as an injection every six months. In contrast, zoledronate is a bisphosphonate that is incorporated directly into the bone, offering a prolonged effect. While both treatments are effective, the discontinuation of denosumab carries the risk of a rebound loss of bone, an issue not associated with zoledronate's mechanism.
Zoledronate vs Zoledronic acid
This is a semantic distinction: zoledronate refers to the ion, while zoledronic acid refers to the molecule. However, in clinical practice, both names are used interchangeably for the exact same therapeutic drug.
Zoledronate vs Pamidronate
Pamidronate is a first‑generation bisphosphonate that’s relatively weak. Zoledronate, by contrast, is more potent, so it can be given in a dose over a shorter infusion while still delivering better skeletal protection.
Zoledronate vs Prolia
Both Prolia (denosumab) and zoledronate treat osteoporosis and bone complications related to cancer, but they work through different mechanisms. Zoledronate is unique because it embeds itself in the bone, providing a long-lasting suppressive effect. In contrast, denosumab requires regular injections to sustain its therapeutic action.
Zoledronate vs Alendronate
Alendronate, a common bisphosphonate for osteoporosis, is taken orally on a regular basis. Zoledronate, however, is given intravenously, allowing for treatments to be scheduled much further apart. The IV formulation is often more beneficial for patients who have trouble tolerating the oral pills or who have issues with gastrointestinal involvement.
Mechanism of Action: How Zolephos Infusion Works
Zolephos Infusion is a member of the bisphosphonate drug class, which is designed to prevent bone loss. These compounds all share a resilient phosphorus-carbon-phosphorus backbone that protects them from being broken down by enzymes. The drug acts with sharp selectivity, targeting the specific bone-remodeling sites where osteoclasts are actively breaking down bone.
Bisphosphonate class mechanism
Zoledronic acid is arguably the most potent member of the bisphosphonate family. Its mechanism involves binding to the hydroxyapatite crystals in the bone. As osteoclasts (bone-resorbing cells) attempt to break down the bone, they engulf the drug. Once inside the cell, zoledronic acid disrupts crucial enzymatic pathways, most notably the mevalonate pathway, which is essential for the osteoclasts' function and survival.
Inhibition of osteoclast-mediated bone resorption
Zoledronate works by interfering with the architecture of osteoclasts—the cells responsible for bone erosion—and pushing them toward apoptosis (cell death), which effectively stops bone loss. This action results in a stronger skeleton, a reduced risk of fractures, and stabilization of the lytic lesions often found in cancer patients.
Effects on calcium metabolism and bone turnover
The Zolephos infusion effectively manages calcium levels by suppressing bone breakdown, thereby limiting the amount of calcium released into the bloodstream. This is a crucial effect for patients suffering from malignancy-driven hypercalcemia (abnormally high calcium due to cancer). Furthermore, the drug significantly reduces key indicators of bone turnover, such as serum alkaline phosphatase and urinary N-telopeptide, reflecting a notable decrease in metabolic activity.
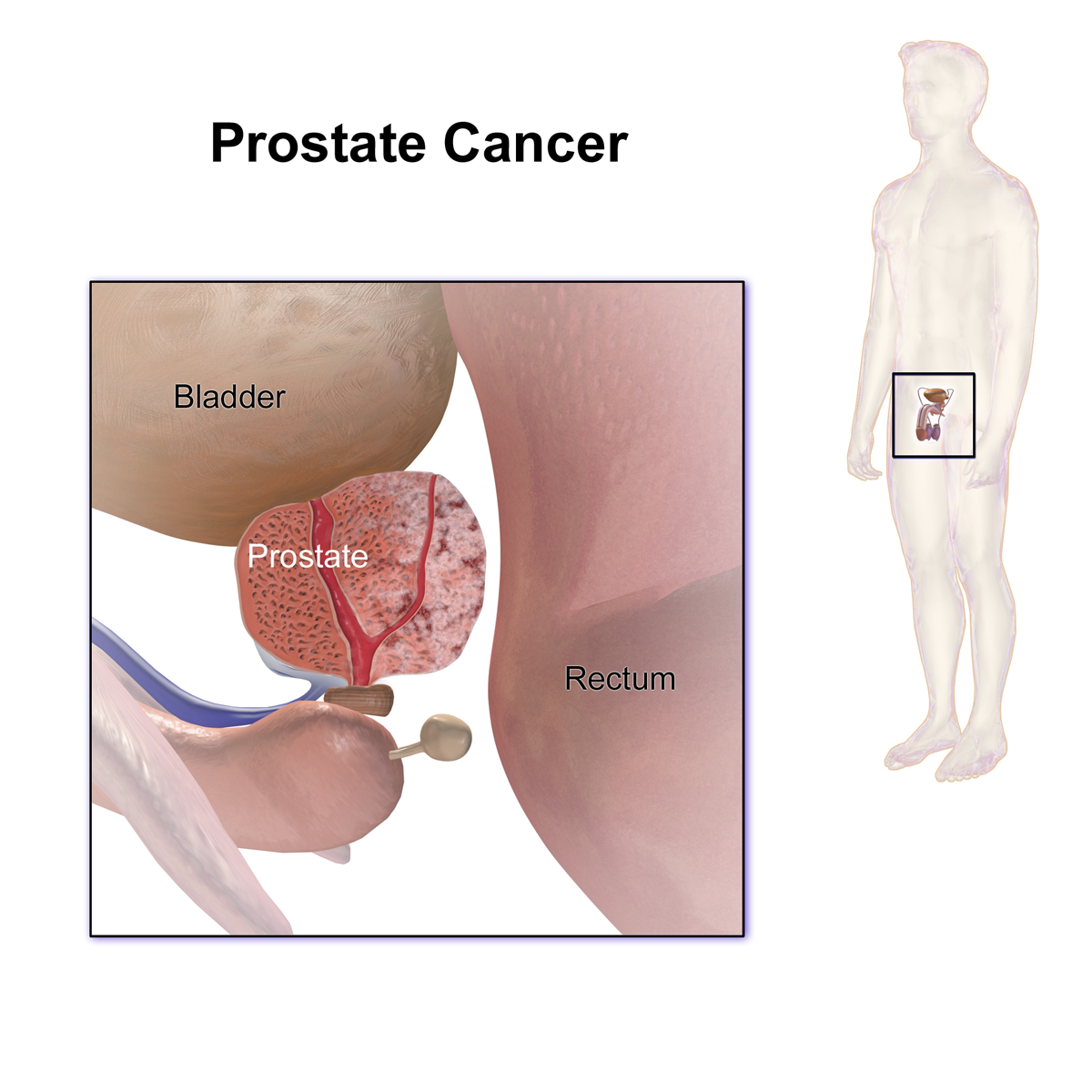
In cancers like breast cancer, prostate cancer, and multiple myeloma, the spread of disease to the bones frequently impairs mobility and worsens the prognosis. Zolephos Infusion counters these effects by helping to maintain bone stability and easing pain. Therefore, its function in oncology is both therapeutic and palliative, improving the patient's quality of life and potentially lengthening the time they remain disease-free.
Approved Medical Uses
Treatment of osteoporosis in postmenopausal women and men
Zolephos Infusion consistently increases bone mineral density in both men and women with osteoporosis. The convenience of its once-yearly dosing schedule significantly enhances patient adherence, and clinical data confirms it reduces the rates of both vertebral and hip fractures.
Prevention of fractures in patients with osteoporosis
Zoledronate significantly strengthens both the internal bone framework and the dense cortical layer, thereby sharply reducing the risk of subsequent fractures. This preventive benefit is especially critical for older adults, as a fragility fracture can rapidly lead to a loss of independence.
Treatment and prevention of glucocorticoid-induced osteoporosis
Zoledronate is approved to counteract the bone loss caused by prolonged corticosteroid therapy. It acts as a protective barrier, reducing the fracture risk for patients who require long-term steroids to manage conditions like asthma or autoimmune disease.
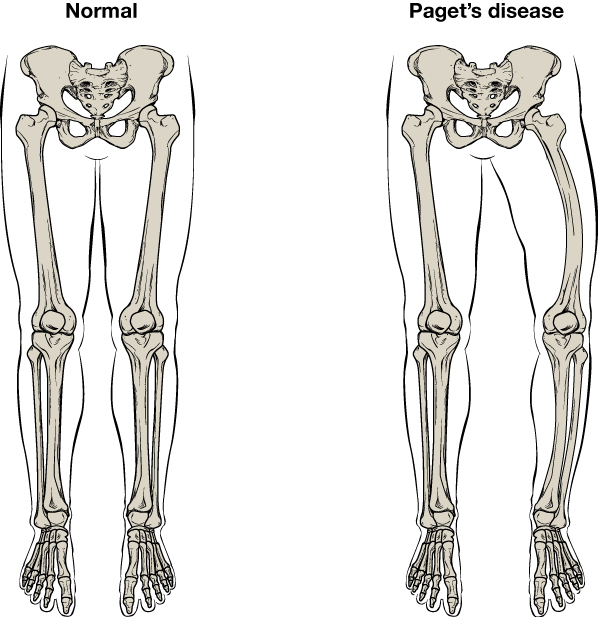
Treatment of Pagets disease of bone
For Paget's disease, which involves abnormal bone remodeling, a single infusion of zoledronate is typically effective. This treatment can keep serum alkaline phosphatase levels regulated for an extended period, signaling that normal skeletal homeostasis has been successfully restored.
Management of bone metastases from solid tumors
When solid tumors like breast and prostate cancer spread to the bone, they cause a host of complications. Delivering the Zolephos Infusion helps mitigate these bone-related problems—including fractures and spinal cord compression—thereby preserving the patient's mobility and improving their quality of life.
Zoledronate not only helps manage the painful and disabling lesions caused by multiple myeloma but may also have a disease-modifying effect by potentially altering the bone-marrow microenvironment.
Zoledronate for hypercalcemia
For patients suffering from malignancy-associated hypercalcemia (abnormally high blood calcium due to cancer), a Zolephos infusion effectively and lastingly reduces serum calcium levels. Because of this potent and sustained response, it is a first-line treatment for this medical emergency.
Zoledronate for breast cancer
When breast cancer metastasizes to the bone, zoledronate is a standard part of the treatment plan because it does more than just reduce bone complications. It also appears to positively influence the metastatic micro-environment, potentially improving outcomes by acting against the tumor itself.
Zoledronate for osteosarcoma in dogs
In veterinary oncology, zoledronate is a key treatment for dogs with osteosarcoma. It works by significantly reducing bone pain and slowing down the destructive lytic breakdown, which helps extend the dog's survival and improve their overall quality of life.
Zoledronate for horses
Veterinarians use zoledronate in horses to treat various skeletal disorders and manage navicular disease. By carefully regulating bone turnover, the drug effectively provides pain relief and can help improve the animal's performance.
Zoledronate for osteogenesis imperfecta
Zoledronate therapy can benefit children and adults with osteogenesis imperfecta (brittle bone disease). This treatment works by reducing the frequency of fractures and increasing bone density, leading to improved mobility and decreased overall illness (morbidity).
Zoledronate for osteosarcoma
Zoledronate is currently being investigated as a therapy for osteosarcoma. Its ability to prevent bone breakdown (anti-resorptive effect) may help limit the disease's spread while also improving skeletal stability during cancer treatment.
Zoledronate for metastatic breast cancer
The Zolephos infusion is a primary treatment for patients with breast cancer because it effectively manages associated bone complications, helping to preserve their mobility and dignity throughout treatment.
Zoledronate for osteopenia
Zoledronate is sometimes administered to individuals with osteopenia (reduced but not yet critical bone mass) to proactively prevent further bone loss. This preventative use highlights the drug's importance across the entire spectrum of bone fragility.
Zoledronate for myeloma
In multiple myeloma, zoledronate's value extends beyond preventing fractures; it actively remodels the bone-marrow environment. By interfering with osteoclast activity, the drug reduces the disease burden and works synergistically with other anti-myeloma treatments.
Off-Label Uses
Hypercalcemia of malignancy beyond standard indications
When standard treatments fail to control hypercalcemia (high blood calcium), clinicians often turn to zoledronate. It rapidly suppresses bone activity, stopping the release of calcium into the blood and typically normalizing serum levels within days. In practice, it is often combined with intravenous fluids and calcitonin for a comprehensive approach to stabilizing the body's internal environment.
- It is most effective against calcium increases driven by the parathyroid hormone-related peptide (PTHrP) syndrome associated with tumors.
- The drug may lengthen the time between hypercalcemia episodes.
- Close monitoring for hypocalcemia (low calcium) and kidney health is essential.
Experimental use in breast cancer prevention of recurrence
Adjuvant (additional) therapies are being investigated for their ability to modify the bone microenvironment, making it less hospitable for cancer cells to establish metastases and potentially preventing the regrowth of dormant tumor cells. While the evidence is not conclusive across all groups, studies hint at a modest reduction in bone recurrences, particularly in post-menopausal patients.
- Benefits appear to be strongly linked to the overall clinical context, making patient selection critical.
- These treatments may act synergistically with endocrine therapy.
- Physicians must carefully weigh the patient's fracture risk, general health status, and kidney function when designing a cancer treatment plan.
Role in prostate cancer bone metastasis management
In castration-resistant prostate cancer, zoledronate is highly effective at stabilizing mixed bone lesions, which helps to reduce pain and lower the frequency of skeletal-related events (SREs). Although it doesn't kill cancer cells directly (it's not cytotoxic), it modifies the bone environment by blocking the osteoclast formation that is triggered by tumor-released substances.
- It adds benefit to standard treatments like androgen-axis therapies and radiopharmaceuticals.
- It is particularly useful when the structural integrity of the spine is at risk.
- Treatment requires monitoring with alkaline phosphatase testing and necessary imaging studies.
Investigational role in chronic kidney disease-associated bone disorders
Renal osteodystrophy presents clinicians with complex bone-turnover patterns. While zoledronate may be considered for patients with high bone turnover, its use requires extreme caution due to the risk of kidney injury and severe disruption to mineral balance. Therefore, mandatory and close collaboration with a nephrologist is essential.
- Creatinine and eGFR must be assessed both at baseline and before each dose.
- Careful management is required for both the replacement of Vitamin D and the control of parathyroid hormone (PTH).
- In this population, the use of extended-interval dosing regimens should be reserved for carefully selected cases.
Dosage and Administration
Standard dosing regimens for osteoporosis
For men and women with osteoporosis, the standard regimen is a single 5 mg intravenous infusion annually. This yearly schedule promotes treatment adherence while providing sustained protection. To prevent low-calcium complications, patients must ensure they have adequate calcium and Vitamin D intake before and after the infusion.
- Patients should be euvolemic (normally hydrated) before receiving the dose.
- Pre-medicating with acetaminophen can help reduce acute-phase reactions.
- A dental evaluation is recommended to minimize the risk of osteonecrosis of the jaw (ONJ).
For managing bone disease and multiple myeloma, the standard regimen is an intravenous infusion of 4 mg every three to four weeks, with the dose modified based on kidney function. The primary goal is to reduce skeletal-related events (SREs), rather than to shrink the tumor directly.
- Consider extending the treatment intervals during long-term maintenance to balance the benefits against potential risks.
- If the patient's creatinine level exceeds established institutional limits, the medication must be held or the dosage adjusted.
- Calcium and Vitamin D supplementation should be continued unless specifically contraindicated.
Infusion preparation and administration guidelines
The correct preparation requires diluting the prescribed dose in 100 mL of either 0.9% saline or 5% dextrose. This mixture must then be infused over a minimum of fifteen minutes using a dedicated IV line or a compatible Y-site. Crucially, calcium-containing solutions must not be mixed with the infusion, as this will cause precipitation.
- Before use, visually inspect the solution for any particles or discoloration.
- Infusing the preparation at room temperature can help minimize patient discomfort.
- After the infusion, flush the line to ensure the entire dose has been delivered.
Duration of therapy and retreatment schedules
The duration of zoledronate treatment is individualized for each patient. For osteoporosis, doctors usually re-evaluate the need for therapy after three to five years, at which point a "drug holiday" may be considered. Conversely, in oncology, treatment generally continues as long as the disease is active or the patient remains at high risk for bone-related complications.
- For Paget's disease, treatment should be restarted if the condition shows signs of relapse, either through symptoms or lab results.
- If the disease remains stable (suppressed), the interval between oncology infusions may be extended.
- Treatment schedules must be adjusted following surgical procedures or if the patient's kidney function changes.
Monitoring requirements during administration
Rigorous pharmacovigilance is central to the treatment protocol. Before each infusion, crucial lab tests, including serum creatinine, calcium, phosphate, and magnesium, must be taken. For the 72 hours following the dose, patients should be monitored for acute-phase reactions such as fever, muscle pain (myalgias), and joint pain (arthralgias).
- In certain patients, bone turnover markers may be useful for tracking treatment effectiveness.
- Regular dental check-ups are necessary over time to help prevent osteonecrosis of the jaw (ONJ).
- If proteinuria or kidney impairment is suspected, a urinalysis should be performed.
Special Populations and Administration Guidance
Administration in elderly patients: dose adjustments and monitoring
Age itself doesn't require a dose reduction; any necessary adjustment is based solely on the patient's kidney function. While older patients benefit from the convenient dosing schedule, they have a higher baseline risk for hypocalcemia (low calcium) and dehydration.
- Closely monitor the estimated Glomerular Filtration Rate (eGFR) and adjust or delay the treatment plan as necessary.
- Ensure adequate dietary calcium and optimize Vitamin D levels.
- Addressing the patient's risk of falls adds significant value by reinforcing the drug's fracture-prevention benefits.
Administration in pregnant women: potential risks and safety data
Because animal studies indicate that bisphosphonates can cross the placenta and potentially harm fetal bone development, and human data is scarce, these drugs should not be used during pregnancy unless the clear therapeutic benefit significantly outweighs the risk.
- Women who can become pregnant should use reliable contraception throughout the treatment period and afterward.
- Pre-conception counseling is recommended for these patients.
- Any new or exceptional use during pregnancy requires a full, documented discussion of the risks and benefits.
Use in nursing mothers: lactation considerations
Animal studies indicate that bisphosphonates can pass through the placenta and could impair fetal bone development. Since human data is limited, routine use during pregnancy is not recommended. Therefore, these drugs should only be used during pregnancy if the potential benefit clearly outweighs the risk to the fetus.
- Patients capable of becoming pregnant are advised to use reliable contraception both during and after the treatment course.
- Pre-conception counseling is important for these patients.
- Any emerging use in pregnancy should involve a complete and documented discussion of the risks and benefits.
Administration in pediatric patients: limited evidence and recommendations
Although data on its use in children is limited, clinicians have gained experience with zoledronate in treating conditions like osteogenesis imperfecta. Establishing appropriate dosing and monitoring for long-term safety—including effects on growth plates and teeth—requires the expertise of a subspecialist.
- The drug should only be used in specialized centers staffed with bone experts.
- It is critical to regularly monitor the child's growth rate, evaluate their bone age, and track biomarkers throughout treatment.
- Given the uncertainties, including parents or guardians in the decision-making process is absolutely necessary.
Warnings and Precautions
Risk of hypocalcemia and supplementation requirements
The Zolephos infusion can cause a drop in calcium levels (hypocalcemia), which is a particular risk for patients with deficiencies in Vitamin D, malabsorption issues, or pre-existing parathyroid problems. Therefore, supplementation with calcium and Vitamin D is crucial. To prevent severe hypocalcemia—which can cause symptoms like muscle spasms (tetany), tingling (paresthesia), or cardiac arrhythmias—patients require close biochemical monitoring.
- Before starting treatment, you must confirm baseline calcium and Vitamin D levels.
- Patients need to continue their supplement regimen throughout the entire course of therapy.
- Seniors and those with pre-existing parathyroid conditions are the highest risk groups.
Osteonecrosis of the jaw (ONJ) risk factors and prevention strategies
Osteonecrosis of the jaw (ONJ) remains a recognized complication of bisphosphonate therapy, with the risk significantly higher in patients undergoing dental surgery, those with poor oral hygiene, or individuals concurrently taking corticosteroids. A sound preventative strategy involves a thorough dental evaluation before starting treatment and avoiding all elective invasive dental procedures while the patient is on the medication.
- Patients must maintain excellent oral hygiene and keep up with routine dental appointments.
- Any necessary procedures, such as tooth extractions or implant placements, should be completed before therapy begins.
- If an invasive dental procedure cannot be avoided, it requires close collaboration with specialists.
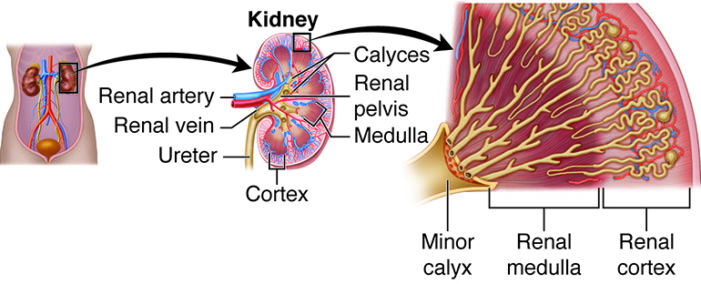
Renal toxicity and monitoring of kidney function
Zoledronate is primarily processed by the kidneys and is known to potentially cause nephrotoxicity, which can manifest as elevated serum creatinine, proteinuria, or even acute renal failure. To mitigate this risk, it is essential to provide hydration before the infusion and adjust the drug dose based on the patient's current kidney function.
- Creatinine clearance must be assessed before every dose.
- Administer the infusion over at least fifteen minutes to reduce stress on the kidney tubules.
- If possible, avoid concurrent use of other nephrotoxic drugs.
Atypical femoral fractures with long-term use
Prolonged use of bisphosphonates has been associated with atypical fractures in the subtrochanteric area and the shaft of the femur. These fractures often present as a persistent ache in the thigh or groin preceding a complete break. Therefore, active surveillance and imaging studies are essential for early detection.
- For osteoporosis patients, a drug holiday can be considered after three to five years of therapy.
- Clinicians should monitor both femurs for any signs of bone changes.
- Decisions must carefully balance the long-term risks against the proven benefits of fracture reduction.
Acute phase reactions post-infusion
Within 72 hours of the infusion, a small number of patients experience transient, flu-like symptoms such as fever, muscle aches, and joint pain. While brief, these acute-phase reactions can negatively affect treatment adherence if patients and clinicians are unprepared.
- The severity of these reactions can often be reduced by a pre-emptive dose of acetaminophen or an NSAID.
- Symptoms typically resolve spontaneously within a few days.
- Subsequent infusions generally result in milder reactions.
Contraindications
Known hypersensitivity to Zoledronate or other bisphosphonates
If a patient develops hypersensitivity reactions—such as a rash or a severe anaphylactic response—it means they must never be exposed to the medication again. Therefore, a complete allergy history must be taken before the drug is administered.
Severe renal impairment and renal failure risks
Zolephos Infusion is generally avoided in patients whose creatinine clearance is below 30 mL/min because they face a high risk of kidney toxicity. The drug should only be used in this severely impaired group if the potential benefits are clearly and exceptionally greater than the risks.
Hypocalcemia at baseline
Before starting therapy, the patient's calcium levels must be normalized. Administering zoledronate when calcium levels are already low can trigger a severe chain of symptomatic electrolyte problems.
Pregnancy and breastfeeding contraindications
Because bisphosphonates can cross the placenta and potentially harm fetal skeletal development, and due to the risk to a nursing infant, Zolephos Infusion is contraindicated (must not be used) during both pregnancy and lactation (breastfeeding).

Careful Administration and Important Precautions
Pre-treatment assessments: renal function, calcium, and vitamin D status
A careful pre-treatment assessment is essential before starting therapy. Clinicians routinely measure serum creatinine and estimate GFR to check kidney function, ensure calcium levels are normal, and confirm that Vitamin D reserves are adequate.
- If a patient's Vitamin D or calcium levels are low, the sensible step is to provide supplementation.
- If kidney function tests are abnormal, the medication dose must be either adjusted or postponed.
Hydration requirements before infusion
To reduce the risk of kidney damage, it's important to ensure adequate hydration. Patients are typically advised to drink fluids before their infusion unless medically prohibited, and those at higher risk may benefit from receiving intravenous fluid support.
Precautions with concomitant dental procedures
Dental procedures significantly increase the risk of osteonecrosis of the jaw (ONJ). Therefore, all elective dental surgeries should be completed before starting therapy. If unavoidable invasive dental work is required during treatment, the doctor and the dentist must collaborate closely.
Monitoring bone density and serum electrolytes during treatment
Regular follow-up visits are essential to confirm the therapy is working and to catch any complications early. Bone mineral density should be re-evaluated every one to two years. Additionally, periodic checks of serum electrolytes (calcium, phosphate, and magnesium) are recommended to prevent imbalances.
- Bone densitometry results guide decisions on the appropriate duration of treatment.
- Monitoring lab results helps mitigate the risks of both low calcium levels and kidney dysfunction.
- Educating patients on potential warning symptoms often leads to earlier detection of complications.
Drug Interactions
Interaction with aminoglycosides and nephrotoxic drugs
Co-administering aminoglycosides (like gentamicin or tobramycin) with zoledronate increases the risk of kidney toxicity and can prolong low calcium levels. This combination stresses the kidney tubules, heightening the risk of acute injury. If both drugs must be used, rigorous monitoring of serum creatinine and electrolyte levels is mandatory.
- Assess kidney function at baseline and monitor it regularly throughout the treatment period.
- If kidney function is impaired, adjust the zoledronate dose or lengthen the interval between doses.
- If possible, use regimens that minimize the combined nephrotoxic impact.
Caution with loop diuretics and risk of hypocalcemia
When loop diuretics, such as furosemide, are used concurrently with a Zolephos infusion, they increase the kidneys' loss of calcium, which can trigger severe hypocalcemia (low calcium). Patients with borderline calcium or Vitamin D reserves are particularly vulnerable to symptoms like irritability and arrhythmias.
- Before starting therapy, ensure the patient has sufficient calcium and Vitamin D in their system.
- It is strongly recommended to monitor serum calcium levels closely when both drugs are used together.
- For especially vulnerable patients, it may be safer to consider switching the diuretic to a different class of medication.
Possible interactions with anti-angiogenic therapies
Using Zolephos Infusion alongside certain anti-angiogenic agents (like bevacizumab or sunitinib) appears to increase the risk of osteonecrosis of the jaw (ONJ). This heightened risk occurs because the combined drugs disrupt the jaw's biology by reducing blood supply and inhibiting normal bone repair, creating conditions ripe for tissue death.
- Before starting treatment, especially with complex regimens, a clearance from a dentist is absolutely critical.
- Patients must consistently maintain excellent oral hygiene throughout the entire course of therapy.
- Remain vigilant for any signs of jaw trouble, such as persistent pain, swelling, or visible exposed bone.

Considerations with concurrent corticosteroid use
Corticosteroids increase the risk of developing osteoporosis and also intensify immunosuppression, which can worsen complications such as jaw osteonecrosis (ONJ) and poor wound healing. Because of these heightened bone-related risks when used with zoledronate, patients require closer monitoring.
- Vitamin D intake should be adjusted to counteract the bone breakdown typically caused by steroid use.
- Dental check-ups must be prioritized for patients receiving both medications.
- When medically possible, it is best to limit the duration of corticosteroid exposure.
Side Effects
Overview of adverse reactions
Side effects from the Zolephos Infusion can affect specific organs or be limited to the injection site. While most of these effects are temporary and manageable, a few can be serious enough to require stopping the infusion or adjusting the dose. Therefore, remaining vigilant and acting quickly is crucial in clinical practice.
After the infusion, or even during it, some patients may experience an acute, flu-like reaction characterized by fever, chills, muscle aches, and joint pain. While these episodes can be uncomfortable, they are usually temporary and tend to resolve on their own within about 72 hours.
Organ-specific toxicities (renal, hepatic, musculoskeletal)
The foremost safety concern is renal toxicity, which typically shows up as a sharp increase in creatinine or, if unaddressed, can lead to kidney failure. Though rare, hepatic (liver) involvement may occur, indicated by changes in liver enzyme levels. Musculoskeletal side effects, such as bone, joint, or muscle pain, can occasionally be severe enough to require discontinuing the treatment.
- Renal: It is crucial to adjust medication doses based on the patient's estimated GFR.
- Hepatic: For high-risk patients, liver enzyme levels should be monitored periodically.
- Musculoskeletal: Patients should be advised to immediately report any severe pain to their care team.
Common Side Effects
Flu-like symptoms: fever, myalgia, arthralgia
A large number of patients experience brief, flu-like symptoms following the infusion. However, these episodes usually become less severe with each subsequent dose, suggesting that the patient's immune system adapts to the medication.
Nausea, vomiting, diarrhea
Gastrointestinal issues are frequently reported, but they are usually mild. Often, discomfort can be relieved with a simple anti-nausea medication or a minor dietary adjustment.
Headache and dizziness
Central nervous system side effects, such as a severe headache or temporary dizziness, are usually short-lived. In these situations, providing proper hydration and using targeted symptom relief typically brings quick and effective comfort.
Fatigue and weakness
Asthenia, or profound weakness/lack of energy, is a symptom that, while often under-reported, can disrupt daily life, particularly in vulnerable patients. When it occurs, supportive care and reassurance are essential.

Local infusion site reactions
Some patients may experience localized redness, swelling, or tenderness at the injection site. This reaction is generally mild, resolves without intervention, and rarely requires stopping the infusion.
- Applying a cold compress to the area typically helps ease the discomfort.
- Whenever feasible, rotating the infusion site reduces the chance of this reaction recurring.
- If a reaction persists or becomes notably severe, it must be evaluated for potential extravasation (leaking of the drug outside the vein).
Overdose and Emergency Management
Potential clinical manifestations of overdose
Overdosing on Zolephos Infusion is dangerous, potentially causing major disruptions to the body's mineral balance and kidney function. The immediate concern is severe hypocalcemia (critically low calcium), which can result in life-threatening events like tetany, seizures, or heart rhythm problems. Further complications include other electrolyte shifts (low phosphate and magnesium), as well as kidney damage or failure. While initial symptoms often involve nausea, vomiting, and abdominal pain, the most severe cases can rapidly advance to cardiovascular collapse and failure of multiple organs.
Immediate medical management strategies
When an overdose occurs, immediate action is crucial. To correct the critically low calcium (hypocalcemia), intravenous calcium gluconate must be administered, carefully adjusted to avoid overcorrecting and causing high calcium (hypercalcemia). Providing ample saline fluids is vital to help the kidneys clear the drug, and diuretics can be carefully added to boost urinary output. Continuous cardiac monitoring is necessary due to the risk of arrhythmias from the electrolyte shifts.
- Start an IV calcium drip immediately to stabilize heart function and calcium levels.
- Administer fluids quickly, unless the patient has a pre-existing heart condition that prevents it.
- If standard treatments fail to improve kidney function, hemodialysis should be considered.
Supportive care and monitoring parameters
Comprehensive supportive care is the foundation for managing an overdose. To guide treatment, it is essential to regularly measure serum levels of calcium, phosphate, magnesium, and creatinine. The core monitoring strategy involves a triad of checks: neurological assessments for seizure activity, cardiac evaluations for arrhythmias, and diligent tracking of kidney function. For complex cases, involving specialists from nephrology, cardiology, and intensive care often leads to better patient outcomes.
Handling and Storage Precautions
Storage conditions for vials and reconstituted solution
For storage, vials should be kept at room temperature and protected from light and excessive humidity. Once the medication is mixed or diluted, the solution should ideally be used immediately. If it cannot be given right away, the prepared infusion must be refrigerated at 2°C to 8°C and used within its established stability timeframe.
Shelf-life and stability considerations
Commercially supplied vials maintain their full strength until the printed expiration date, provided they are stored correctly. Once the drug is mixed (reconstituted), the resulting solution has limited stability; storing it for too long risks compromising its sterility and reducing its effectiveness. Strict adherence to the manufacturer's guidelines is necessary to ensure the drug performs as intended.
- Any reconstituted product remaining unused past the recommended time limit must be discarded.
- Do not allow the solution to freeze, as ice crystals can compromise the drug's quality.
- Before giving the infusion, visually inspect the preparation for any cloudiness or changes in color.
Safe handling and disposal practices
A meticulous approach is required when handling Zolephos Infusion to ensure the safety of both staff and patients. Proper personal protective equipment (PPE) must be used during both preparation and administration. If a spill occurs, it should be cleaned up using cytotoxic-handling procedures, even though zoledronate is not formally classified as a cytotoxic agent. Finally, all unused product and related waste must be disposed of according to environmental regulations.

Guidelines for healthcare professionals in preparation
To maintain sterility, clinicians must use strict aseptic technique when preparing and diluting the medication. Infusion lines must be dedicated or proven compatible to prevent the medication from mixing with other solutions and causing precipitation. Thorough training on preparing the infusion and recognizing potential adverse events is key to both patient safety and consistent therapeutic results across different healthcare environments.
- Always confirm the medication's name, strength, and volume before administration.
- Mix solutions in a clean environment using sterilized equipment.
- Recording the lot number and administration time of each dose makes tracing the medication easy.
Zolephos Infusion, Zoledronate FAQ
- How is Zoledronate administered?
- How does Zoledronate infusion work?
- What is Zoledronate infusion?
- What is Zoledronate infusion used for?
- What is Zoledronate injection used for?
- What is Zoledronate used for?
- What does Zoledronate infusion do?
- What is Zoledronate treatment?
- Can Zoledronate be purchased over the counter?
- Zoledronate for dogs?
- Zoledronate for osteoporosis?
- Zoledronate for hypercalcemia?
- Zoledronate for breast cancer?
- Zoledronate for cats?
- Zoledronate for osteosarcoma in dogs?
- Zoledronate for horses?
- Zoledronate for osteogenesis imperfecta?
- Zoledronate for osteosarcoma?
- Zoledronate for metastatic breast cancer?
- Zoledronate for osteoporosis dose?
- Zoledronate for osteopenia?
- Zoledronate for myeloma?
- Zoledronate for dogs side effects?
- Is Zoledronate a bisphosphonate?
- Is Zoledronate infusion safe?
- Is Zoledronate cytotoxic?
- What is Zoledronate?
- Zoledronate how to use?
- Zoledronate, how to give?
- Zoledronate for hypercalcemia?
- Zoledronate for dental work?
- Zoledronic or Zoledronate?
- Pamidronate or Zoledronate?
- Zoledronate for arthritis?
- Zoledronate vs Alendronate?
- Zoledronate vs Prolia?
- Zoledronate vs Pamidronate?
- Zoledronate vs Zoledronic acid?
- Zoledronate vs Denosumab?
- Zoledronate vs Risedronate?
- Zoledronate vs Ibandronate?
- Zoledronate vs Denosumab for osteoporosis?
- Zoledronate vs Reclast?
- Zoledronate vs Zoledronic?
- Aclasta vs Zoledronate?
- What is Zoledronate brand name?
- What is Zoledronate bisphosphonate?
- Zoledronate for breast cancer?
How is Zoledronate administered?
In a clinical setting, zoledronate is delivered as an IV infusion through a vein, in the arm and the drip runs for at least fifteen minutes. However, there is also an infusion version of this product
How does Zoledronate infusion work?
Zoledronate, given as an infusion latches onto bone shutting down the osteoclasts that chew it away; this slows the loss of bone. Can actually preserve or even raise bone density.
What is Zoledronate infusion?
Zoledronate infusion delivers the medication into the veins letting the bloodstream absorb it in a controlled manner.
What is Zoledronate infusion used for?
Zoledronate infusion treats osteoporosis, it helps prevent fractures in patients whose cancer has spread to bone, controls hypercalcemia, and addresses a variety of bone‑related conditions.
What is Zoledronate injection used for?
Doctors prescribe injections for a handful of bone‑related troubles—osteoporosis Paget’s disease, cancer‑related bone complications and the dangerous rise in blood calcium that can accompany malignancies.
What is Zoledronate used for?
Zoledronate is employed to boost bone density, cut the odds of fractures, ease bone‑related pain, and to manage complications that arise when cancer spreads to bone and bring down calcium levels in the bloodstream.
What does Zoledronate infusion do?
A zoledronate infusion typically dampens bone loss, boosts bone mineral density, trims fracture risk and helps stabilize calcium levels in the bloodstream.
What is Zoledronate treatment?
The treatment plan, for zoledronate revolves around scheduled IV infusions— an annual dose for osteoporosis though more frequent dosing is common, for bone issues linked to cancer.
Can Zoledronate be purchased over the counter?
No—Zoledronate can’t be bought over the counter; it requires a physician’s prescription and clinical monitoring, so it isn’t sold as an OTC medication.
Zoledronate for dogs?
In some contexts, zoledronate is used to ease bone pain or combat conditions such as osteosarcoma in dogs. Its application is strictly off‑label and must be overseen by a veterinarian.
Zoledronate for osteoporosis?
Zoledronate has become a staple, in osteoporosis treatment curbing bone‑resorbing activity and dramatically slashing fracture risk, for women and for men deemed high‑risk.
Zoledronate for hypercalcemia?
Zoledronate is employed to manage hypercalcemia acting to curb calcium leaching from bone and steer serum calcium back, toward levels.
Zoledronate for breast cancer?
In breast cancer management zoledronate is deployed to preempt complications arising from bone metastases and to diminish fracture risk.
Zoledronate for cats?
Zoledronate has found its way into cat studies as a balm for bone agony or as a weapon against cancer‑linked troubles; still, it lives in the experimental zone and calls for a vet’s careful oversight.
Zoledronate for osteosarcoma in dogs?
Zoledronate may be prescribed to dogs with osteosarcoma to relieve bone pain and slow bone destruction ,albeit as an off‑label use.
Zoledronate for horses?
Approved for equine use, under different labels, zoledronate is employed to treat navicular syndrome and other bone‑related lameness conditions.
Zoledronate for osteogenesis imperfecta?
When overseen by specialists, zoledronate can help people with osteogenesis imperfecta strengthen their bones, experience fractures, and enjoy a quality of life.
Zoledronate for osteosarcoma?
Although it isn’t a frontline cure, zoledronate often finds its way into the care toolbox for osteosarcoma, where it helps bone destruction ease pain and keep skeletal complications at bay.
Zoledronate for metastatic breast cancer?
In breast cancer zoledronate is often used to lower the chance of problems—fractures, spinal‑cord compression and the bone pain that usually comes with bone metastases.
Zoledronate for osteoporosis dose?
When treating osteoporosis the standard approach is a 5‑mg Zoledronate IV infusion, delivered over least 15 minutes under medical supervision.
Zoledronate for osteopenia?
Zoledronate isn’t a choice for osteopenia. In patients deemed high‑risk, it can be prescribed to keep the condition from slipping into osteoporosis and to lower the chance of fractures.
Zoledronate for myeloma?
When dealing with myeloma, zoledronate often works to prevent bone complications alleviate pain and bring down the risk of fractures caused by lytic bone lesions.
Zoledronate for dogs side effects?
In dogs Zoledronate may give rise to kidney impairment, reduced calcium, gastrointestinal irritation, and on occasions jaw complications; consequently its administration calls for close vigilant veterinary monitoring.
Is Zoledronate a bisphosphonate?
In fact, zoledronate, when given intravenously is a bisphosphonate that curtails bone resorption by acting directly on osteoclasts.
Is Zoledronate infusion safe?
Although a zoledronate infusion administered correctly is broadly regarded as safe, it does harbour a suite of effects—ranging from flu‑like malaise and hypocalcaemia to renal impairment and, in rare instances osteonecrosis of the jaw—necessitating vigilant monitoring, throughout treatment.
Is Zoledronate cytotoxic?
Even though zoledronate isn’t technically classified as a chemotherapy drug, it still pulls duty—damping bone resorption and curbing tumor growth right within the bone microenvironment.
What is Zoledronate?
Administered intravenously, zoledronate is a bisphosphonate that treats osteoporosis, Paget’s disease, hypercalcemia of malignancy and bone complications arising from cancer.
Zoledronate how to use?
Zoledronate is administered via infusion— in a hospital or clinic setting—, with both the dose and the dosing interval tailored to the underlying condition.
Zoledronate, how to give?
Zoledronate is given as an IV infusion lasting at 15 minutes making sure the patient stays well‑hydrated and has adequate calcium and vitamin D levels.
Zoledronate for hypercalcemia?
Zoledronate proves effective, against malignancy‑associated hypercalcemia cutting bone resorption and pulling calcium down, from the bloodstream.
Zoledronate for dental work?
Because Zoledronate can trigger osteonecrosis of the jaw, a dental examination before starting the drug is advisable; whenever feasible, invasive dental work should be kept to a minimum during therapy.
Zoledronic or Zoledronate?
Zoledronic acid and Zoledronate are two monikers for the acid the label most frequently encountered in clinical settings while Zoledronate' serves as its formal chemical name.
Pamidronate or Zoledronate?
While pamidronate and zoledronate both belong to the bisphosphonate family, zoledronate’s potency and its ability to be given in a shorter infusion often make it the favored choice, in many clinical settings.
Zoledronate for arthritis?
Zoledronate isn’t an arthritis treatment; however, for those battling both arthritis and osteoporosis, it can help by lowering fracture risk and reinforcing bone.
Zoledronate vs Alendronate?
Both Zoledronate and Alendronate fall under the bisphosphonate umbrella yet their administration routes differ markedly: Zoledronate arrives as an infusion while Alendronate is an oral preparation taken on a weekly—or, in some regimens daily—basis.
Zoledronate vs Prolia?
Zoledronate belongs to the bisphosphonate class whereas Prolia (denosumab) is a monoclonal antibody. In patients, with function zoledronate is generally the preferred agent while Prolia is often turned to when bisphosphonates are unsuitable.
Zoledronate vs Pamidronate?
Both agents are bisphosphonates. Zoledronate proves more potent, needs a briefer infusion and ends up being the drug clinicians reach for more often in oncology and osteoporosis settings.
Zoledronate vs Zoledronic acid?
Zoledronate and zoledronic acid are one and the same—“zoledronic acid" is the name used for the drug, while "zoledronate" denotes the chemical salt form of that compound.
Zoledronate vs Denosumab?
Zoledronate, by virtue of its bisphosphonate properties, dampens bone resorption while Denosumab takes a route inhibiting RANKL; each cuts the odds of a fracture. When renal function is compromised, clinicians tend to lean toward Denosumab.
Zoledronate vs Risedronate?
Zoledronate is delivered via IV on a schedule, whereas Risedronate is a bisphosphonate taken on a weekly or monthly cadence imposing rigorous compliance with its administration protocols.
Zoledronate vs Ibandronate?
Both belong to the bisphosphonate family. Zoledronate is a potent drug and tends to be used less often, while Ibandronate can be administered either orally or intravenously though data on its role in cancer‑related bone disease are more limited.
Zoledronate vs Denosumab for osteoporosis?
Both drugs work well for osteoporosis, yet Zoledronate is administered once a year by infusion while Denosumab is given as an injection every six months; the decision typically hinges on kidney function, how convenient the schedule is and the risk of rebound bone loss.
Zoledronate vs Reclast?
Reclast is simply the brand name of acid, so zoledronate and Reclast are, in effect the same medication.
Zoledronate vs Zoledronic?
Both terms denote that the acid is the formal official name, while Zoledronate is the version most commonly used.
Aclasta vs Zoledronate?
Aclasta is the tag, for acid a drug chiefly used to fight osteoporosis and Paget’s disease. When the branding is stripped off the same molecule is simply called zoledronate.
What is Zoledronate brand name?
In pharmacies Zoledronate generally appears under the labels Reclast or Zometa although in regions it’s also found as Aclasta.
What is Zoledronate bisphosphonate?
When given intravenously zoledronate works as a bisphosphonate, halting bone resorption and leaving the skeleton noticeably stronger.