1. Introduction
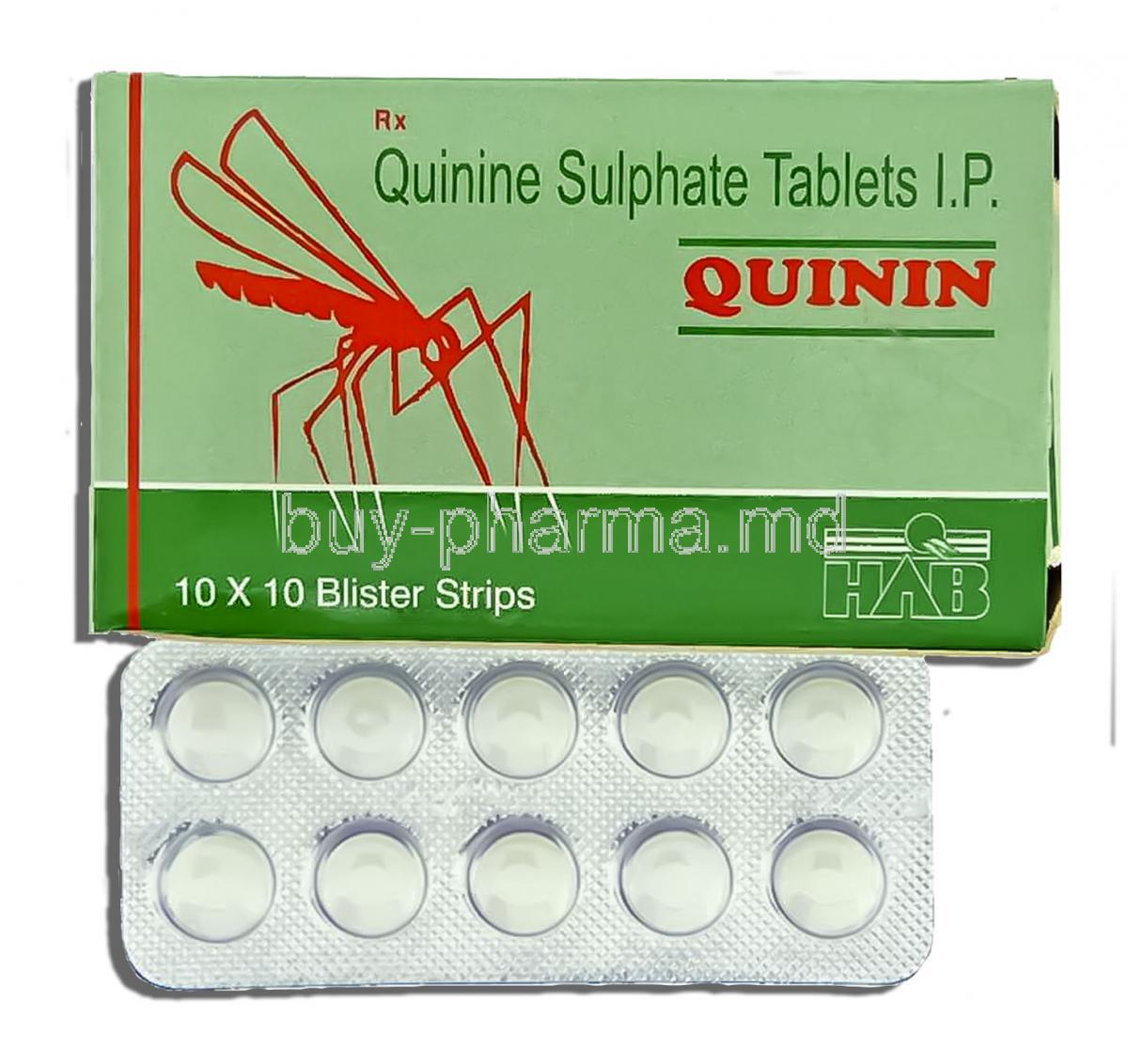
1.1 Overview of Quinin (Quinine Sulphate)
Quinin, known scientifically as Quinine Sulphate, is a well-established alkaloid derived from the bark of the Cinchona tree. It is primarily used as an antimalarial agent, recognized for its potent activity against *Plasmodium* species, particularly *Plasmodium falciparum*. In addition to its therapeutic role in malaria, Quinine has historically been utilized in treating nocturnal leg cramps and muscle hyperexcitability disorders. Its complex pharmacological profile and narrow therapeutic index necessitate careful clinical supervision.
1.2 Historical Background and Medical Significance
The use of Quinine dates back to the 17th century, when it was introduced to Europe as a miraculous “Jesuit’s bark” remedy for fevers. For centuries, it remained the cornerstone of malaria treatment before the advent of synthetic antimalarials. Despite the development of newer agents, Quinine retains clinical relevance in resistant malaria cases and continues to symbolize the foundation of tropical medicine.
1.3 Classification and Pharmacological Category
Quinine Sulphate belongs to the class of antimalarial alkaloids and is categorized pharmacologically as a blood schizonticide. It also exhibits mild analgesic, antipyretic, and muscle relaxant properties. Its mechanism differs from other modern agents, providing a crucial alternative in multidrug-resistant malaria management.
1.4 Regulatory Status and Global Use
Quinine is included in the World Health Organization’s List of Essential Medicines due to its efficacy and medical necessity. It is approved by regulatory bodies such as the FDA and EMA for specific malaria indications. However, its use for leg cramps has been restricted or withdrawn in several countries due to safety concerns related to toxicity and adverse effects.
2. Composition and Formulation
2.1 Active Ingredient: Quinine Sulphate
The principal active component, Quinine Sulphate, is a white crystalline powder with a bitter taste and high solubility in acidic solutions. It acts by interfering with the parasite’s metabolic pathways during the erythrocytic stage.
2.2 Available Dosage Forms (Tablets, Capsules, Injections)
- Oral tablets or capsules for uncomplicated malaria
- Intravenous and intramuscular preparations for severe or cerebral malaria
- Combination formulations with doxycycline or clindamycin for enhanced efficacy
2.3 Strengths and Pharmaceutical Preparations
Typical strengths include 200 mg and 300 mg tablets, standardized to Quinine Sulphate. Parenteral forms vary in concentration depending on clinical indication and setting.
2.4 Inactive Ingredients and Excipients
Formulations may contain lactose monohydrate, magnesium stearate, povidone, and cellulose derivatives. These inactive compounds ensure stability and bioavailability while maintaining dosage uniformity.
3. Mechanism of Action – How Quinine Works
3.1 Pharmacodynamics and Mode of Action in Malaria
Quinine targets the asexual erythrocytic stages of *Plasmodium* by inhibiting heme polymerization. The drug converts toxic heme, released during hemoglobin digestion, into a harmless crystalline form. Disruption of this process causes toxic accumulation and parasite death.
3.2 Interaction with Hemoglobin Metabolism in Plasmodium
Within infected erythrocytes, Quinine interferes with parasite digestion of hemoglobin, resulting in oxidative damage. This process is essential in eradicating parasites from systemic circulation.
3.3 Effects on Muscle Excitability and Neuromuscular Transmission
Quinine depresses the excitability of motor endplates and reduces muscle spasm frequency. These neuromuscular effects underlie its former use in nocturnal leg cramp therapy.
3.4 Central Nervous System and Cardiac Effects
At therapeutic levels, Quinine mildly stimulates the CNS and depresses cardiac excitability. However, excessive plasma concentrations can precipitate arrhythmias or neurological toxicity.
4. Therapeutic Uses
4.1 Approved Medical Uses
4.1.1 Treatment of Uncomplicated Malaria
Indicated for *Plasmodium falciparum* and *P. vivax* infections, Quinine is effective where chloroquine resistance is prevalent. It is often used in combination with doxycycline, clindamycin, or primaquine to prevent relapse.
4.1.2 Adjunctive Use in Severe Malaria
In critical malaria cases, intravenous Quinine serves as a life-saving therapy until patients can tolerate oral medication.
4.1.3 Role in Malaria Prophylaxis (Historical Use)
Historically, Quinine was used for prophylaxis among travelers in endemic regions, though it has largely been replaced by safer alternatives.
4.2 Off-Label and Investigational Uses
4.2.1 Nocturnal Leg Cramps
Once commonly prescribed for nighttime muscle cramps, its use has declined due to safety concerns related to cardiac and hematologic toxicity.
4.2.2 Myotonia and Muscle Spasms
In rare cases, Quinine is used under medical supervision for myotonic disorders where alternative therapies are ineffective.
4.2.3 Babesiosis
When combined with clindamycin, Quinine demonstrates effectiveness against *Babesia microti* infections in immunocompromised individuals.
4.2.4 Tinnitus and Inner Ear Disorders
Historically, Quinine was explored for auditory conditions such as tinnitus, though these uses have become obsolete due to the risk of ototoxicity.
5. Dosage and Administration
5.1 Standard Adult Dosing for Malaria
The typical oral dose ranges from 600 to 650 mg every 8 hours for 3 to 7 days, depending on the infection severity and region-specific resistance patterns.
5.2 Pediatric Dosage Recommendations
Children receive approximately 8 mg/kg body weight every 8 hours, adjusted to age, weight, and tolerance.
5.3 Intravenous vs. Oral Administration
Intravenous Quinine is reserved for severe cases when oral intake is not feasible. Infusion must be slow and diluted to prevent cardiac complications.
5.4 Duration of Therapy and Combination Regimens
Treatment duration typically spans 7 days. Combination therapy with tetracyclines or macrolides enhances parasite clearance and prevents recrudescence.
5.5 Dose Adjustments in Hepatic or Renal Impairment
Reduced dosing intervals are essential for patients with hepatic or renal insufficiency due to altered drug clearance.
5.6 Missed Dose and Discontinuation Guidance
If a dose is missed, it should be taken promptly unless nearing the next scheduled dose. Abrupt discontinuation can result in incomplete parasite eradication.
6. Pharmacokinetics
6.1 Absorption and Bioavailability
Quinine is rapidly absorbed from the gastrointestinal tract, reaching peak plasma levels within 1 to 3 hours post-administration. Food enhances absorption and reduces gastrointestinal irritation.
6.2 Distribution and Plasma Protein Binding
Approximately 70–90% of Quinine binds to plasma proteins, allowing extensive tissue distribution, including placental and cerebrospinal compartments.
6.3 Metabolism (Role of CYP3A4 and Other Enzymes)
The drug undergoes hepatic biotransformation primarily through the CYP3A4 pathway. Metabolites are pharmacologically inactive.
6.4 Elimination and Half-Life
The elimination half-life is approximately 11 hours, with renal excretion accounting for most clearance.
6.5 Factors Influencing Pharmacokinetics
- Concurrent use of CYP inhibitors or inducers
- Renal or hepatic dysfunction
- Age, pregnancy, and comorbid conditions
7. Side Effects and Adverse Reactions
7.1 Overview of Potential Adverse Effects
Quinine’s therapeutic window is narrow, and side effects are dose-dependent. Patients must be closely monitored for early toxicity signs.
7.2 Common Side Effects
- Nausea, vomiting, and diarrhea
- Tinnitus and hearing impairment
- Headache and dizziness
- Blurred or altered vision
7.3 Serious and Rare Adverse Reactions
- Cinchonism: A toxic syndrome marked by tinnitus, visual changes, and gastrointestinal distress
- Cardiac arrhythmias and QT prolongation
- Hypoglycemia, particularly in pregnant patients
- Hemolytic anemia and thrombocytopenia
- Severe allergic reactions including anaphylaxis
8. Drug Interactions
8.1 Interactions with Antimalarial Agents
Concurrent use with mefloquine or chloroquine may enhance cardiac toxicity. Careful combination planning is necessary.
8.2 CYP Enzyme-Induced Drug Interactions
Drugs that inhibit CYP3A4, such as ketoconazole, can elevate Quinine plasma levels, increasing toxicity risk.
8.3 Interactions with Cardiac Medications
Concurrent administration with digoxin or quinidine may potentiate arrhythmic risk.
8.4 Interactions with Anticoagulants and Antibiotics
Quinine may augment warfarin effects and alter antibiotic metabolism, necessitating dosage adjustments.
8.5 Alcohol and Food Interactions
Alcohol may intensify CNS side effects and should be avoided during therapy.
9. Warnings and Important Precautions
9.1 Risk of Cinchonism and Monitoring Parameters
Early symptoms include tinnitus, vertigo, and blurred vision. Monitoring plasma concentration is crucial for prolonged therapy.
9.2 Cardiac Monitoring for QT Prolongation
Electrocardiographic surveillance is required, especially when co-administered with other QT-prolonging drugs.
9.3 Risk of Hypoglycemia, Especially in Pregnant Women
Quinine stimulates pancreatic insulin release, which may precipitate hypoglycemia in susceptible individuals.
9.4 Neurological and Visual Disturbances
Visual impairment, diplopia, and auditory disturbances can indicate early toxicity requiring dose modification or discontinuation.
9.5 Avoidance in G6PD Deficiency and Myasthenia Gravis
Quinine may induce hemolysis in glucose-6-phosphate dehydrogenase–deficient individuals and exacerbate muscle weakness in myasthenia gravis.
10. Contraindications
10.1 Known Hypersensitivity to Quinine or Related Alkaloids
Quinine is contraindicated in individuals with a known hypersensitivity to quinine, quinidine, or other cinchona alkaloids. Allergic manifestations may include rash, pruritus, angioedema, and in severe cases, anaphylactic shock. Patients with a history of such reactions must avoid all formulations containing quinine to prevent recurrence of hypersensitivity-related complications.
10.2 History of Blackwater Fever
Individuals with a prior episode of Blackwater Fever—a rare but serious hemolytic reaction associated with quinine—should not receive the medication again. This condition involves massive hemolysis, hemoglobinuria, and potential renal failure, posing a substantial risk upon re-exposure.
10.3 Optic Neuritis or Retinal Disorders
Quinine is contraindicated in patients with pre-existing optic neuritis or significant retinal pathology. Its use can exacerbate visual disturbances or induce irreversible optic damage, particularly at high plasma concentrations.
10.4 Myasthenia Gravis and Prolonged QT Syndrome
Patients with Myasthenia Gravis may experience worsening muscle weakness due to quinine’s neuromuscular blocking effects. Similarly, individuals with prolonged QT interval or pre-existing cardiac conduction disorders face elevated risks of arrhythmias, syncope, and sudden cardiac arrest when administered quinine.
10.5 Hemolytic Conditions or Thrombocytopenia
Quinine should not be used in patients with hemolytic anemia, thrombocytopenia, or immune-mediated hematologic disorders. Its potential to trigger immune thrombocytopenic purpura (ITP) and hemolysis warrants strict exclusion in such conditions.
11. Careful Administration and Monitoring
11.1 Monitoring Blood Glucose and ECG
Continuous monitoring of blood glucose is recommended, especially in pregnant women, as quinine can provoke hypoglycemia by stimulating insulin secretion. Electrocardiographic (ECG) monitoring is essential to detect QT prolongation and arrhythmic tendencies during therapy.
11.2 Liver and Kidney Function Tests During Therapy
Periodic hepatic and renal assessments are vital. Quinine undergoes hepatic metabolism and renal excretion, making dose adjustment crucial in impaired organ function. Elevations in liver enzymes or signs of nephrotoxicity warrant immediate intervention.
11.3 Dose Adjustment in Chronic Illness or Co-medication
In patients with chronic illnesses such as heart failure, diabetes, or hepatic insufficiency, cautious titration of dose is required. Drug-drug interactions involving CYP3A4 modulators or cardiac depressants must be carefully evaluated before initiation.
11.4 Caution with Elderly and Debilitated Patients
Elderly individuals exhibit altered pharmacokinetics and increased susceptibility to toxicity. Lower starting doses and enhanced monitoring are advisable to prevent adverse neurological or cardiovascular outcomes.
12. Administration in Special Populations
12.1 Use in Elderly Patients
Pharmacokinetic Considerations and Risk of Toxicity: Aging reduces hepatic metabolism and renal clearance, resulting in prolonged plasma half-life. These physiological changes necessitate dose moderation to avoid accumulation.
Adjusted Dosing and Close Monitoring: Regular assessment of auditory and visual function, glucose levels, and ECG readings ensures early detection of adverse responses. Adverse effects such as dizziness or hypotension may predispose elderly patients to falls and injuries.
12.2 Use in Pregnant Women and Nursing Mothers
Safety Profile During Pregnancy: Quinine is considered effective and relatively safe for malaria treatment during pregnancy when benefits outweigh risks. It remains one of the few options for managing multidrug-resistant malaria in endemic regions.
Fetal Effects and Placental Transfer: Quinine readily crosses the placenta and may induce transient fetal hypoglycemia or uterine contractions. Close supervision is required during treatment to mitigate risks.
Excretion in Breast Milk and Neonatal Considerations: The drug is excreted in small amounts in breast milk. Although not typically harmful to the infant, monitoring for signs of irritability or feeding difficulties is advisable.
12.3 Use in Pediatric Patients
Safety and Efficacy Data in Children: Quinine remains a therapeutic option for pediatric malaria; however, careful dosing is crucial due to variable drug clearance and heightened risk of toxicity.
Recommended Pediatric Dosage and Monitoring: Dosing typically ranges from 8 mg/kg every 8 hours. Children should be observed for signs of cinchonism—tinnitus, nausea, and visual changes—as these may indicate excessive serum levels.
13. Overdosage and Toxicity
13.1 Symptoms of Acute Quinine Toxicity
- Severe Cinchonism: Marked by tinnitus, blurred vision, nausea, and confusion.
- Cardiac Arrhythmia and Hypotension: Resulting from excessive myocardial depression or QT prolongation.
- Seizures and Respiratory Distress: Manifestations of central nervous system toxicity in critical overdoses.
13.2 Emergency Management of Overdose
- Gastric Lavage and Activated Charcoal: Early decontamination reduces absorption in acute ingestion cases.
- Supportive and Symptomatic Treatment: Fluid resuscitation and vasopressors may be needed to stabilize hemodynamics.
- Cardiac and Neurological Monitoring: Continuous ECG and neurological surveillance are mandatory until clinical stability is achieved.
14. Handling and Storage Precautions
14.1 Recommended Storage Conditions (Temperature, Light, Moisture)
Store Quinine Sulphate in a cool, dry environment at temperatures below 25°C. Protect from light exposure and humidity to maintain chemical integrity.
14.2 Stability and Shelf Life
Stable under recommended storage for up to three years. Discoloration or changes in odor indicate degradation, warranting disposal.
14.3 Safe Handling Instructions for Healthcare Professionals
Healthcare personnel should handle the medication with clean, dry hands or gloves. Avoid contact with mucous membranes and ensure accurate dosage measurement, especially for injectable formulations.
14.4 Disposal Guidelines for Expired or Unused Medication
Expired quinine should be disposed of following pharmaceutical waste protocols. Do not flush into drainage systems; return to authorized collection points for safe destruction.
15. Clinical Considerations and Patient Counseling Information
15.1 Importance of Completing Full Treatment Course
Patients must complete the entire prescribed course, even after symptom resolution, to ensure full parasite eradication and prevent recurrence or resistance development.
15.2 Avoiding Self-Medication and Monitoring for Adverse Effects
Unsupervised use of quinine for non-malarial conditions is strongly discouraged. Patients should immediately report symptoms such as ringing in the ears, dizziness, or vision disturbances.
15.3 Guidance on Recognizing Early Signs of Toxicity
- Persistent nausea or vomiting
- Hearing loss or tinnitus
- Irregular heartbeat or fainting
- Unusual bleeding or bruising
Prompt medical evaluation is imperative upon the appearance of these symptoms.
15.4 Patient Education for Preventing Malaria Recurrence
Educating patients on mosquito prevention measures—such as bed nets, repellents, and prophylactic drugs—is critical for sustained protection. Reinfection can occur in endemic zones without continued vigilance.
16. Summary and Key Takeaways
16.1 Therapeutic Importance of Quinine Sulphate
Quinine Sulphate remains a cornerstone in the management of resistant malaria strains. Its efficacy in eliminating *Plasmodium falciparum* continues to uphold its significance in global health practice.
16.2 Balance Between Efficacy and Safety
The therapeutic value of quinine is counterbalanced by its narrow safety margin. Clinicians must weigh benefits against risks, ensuring precise dosing and continuous monitoring to minimize adverse outcomes.
16.3 Role in Modern Antimalarial Therapy and Future Prospects
Although supplanted by newer agents in many regions, quinine endures as a vital second-line therapy and an important benchmark for antimalarial pharmacology. Ongoing research into optimized formulations may expand its safe use and preserve its historical legacy in modern medicine.
Quinin, Quinine Sulphate FAQ
- What is quinine sulfate used for?
- Is quinine sulfate the same as quetiapine?
- When is the best time to take quinine sulfate?
- What fruits contain quinine?
- What can I take instead of quinine sulphate?
- What are the benefits of quinine?
- Who cannot take quinine?
- What disease is cured by quinine?
- What are the side effects of quinine sulfate?
- What not to mix with quinine?
- How long does quinine stay in your system?
- Is quinine in Gatorade?
- When is the best time to take quinine sulphate?
- Is quinine good for arthritis?
- Which disease is cured by quinine?
- Can quinine damage kidneys?
- What are the healing properties of quinine?
- Does quinine make you sleepy?
- Is quinine bad for your liver?
- How many days is quinine given?
What is quinine sulfate used for?
For years, a bedtime dose of 200–300 mg quinine sulfate has been employed to ease leg cramps. These idiopathic cramps are a nuisance, especially among older individuals. The drug is believed to work by lowering the excitability of the motor end‑plate and lengthening the muscle’s refractory period.
Is quinine sulfate the same as quetiapine?
Quetiapine is commonly prescribed for disorders and schizophrenia illnesses related to schizophrenia that often first surface in adulthood. Quinine, in contrast, is used to soothe cramps—a complaint that tends to be more prevalent among adults.
When is the best time to take quinine sulfate?
Bedtime
What fruits contain quinine?
Grapefruit
What can I take instead of quinine sulphate?
- Amines
- Calcium Channel Blockers
- Cyclohexanecarboxylic Acids
- Muscle Relaxants, Central
- Vitamin E
- Carisoprodol
- Gamma-Aminobutyric Acid
- Gabapentin
What are the benefits of quinine?
Quinine is the go‑to drug for malaria that springs from Plasmodium falciparum. This parasite sneaks into the body’s blood cells setting off the malaria symptoms. By either wiping out the bug or halting its replication, quinine does its job.
Who cannot take quinine?
- If you’ve ever experienced an irregular heartbeat, have low potassium levels in your blood, or suffer from heart, kidney, or liver disease.
What disease is cured by quinine?
Quinine is a medication that treats malaria.
What are the side effects of quinine sulfate?
- nausea
- vomiting
- diarrhea
- stomach pain
- headache
What not to mix with quinine?
- Astemizole
- Aurothioglucose
- Bepridil
- Cisapride
- Dronedarone
- Eliglustat
- Fluconazole
- Ketoconazole
How long does quinine stay in your system?
96 hours
Is quinine in Gatorade?
Yes
When is the best time to take quinine sulphate?
Nightime
Is quinine good for arthritis?
Quinine carries analgesic and anti‑inflammatory properties. It has found application in the management of arthritis, systemic lupus erythematosus (SLE), and the dreaded nocturnal leg cramps.
Which disease is cured by quinine?
Malaria and babesiosis
Can quinine damage kidneys?
Yes
What are the healing properties of quinine?
Quinine has long been used to combat malaria and its accompanying fevers, to soothe leg cramps caused by spasms, to treat hemorrhoids, to ease varicose veins, and to manage pleural cavity issues after thoracoplasty.
Does quinine make you sleepy?
Quinine can lead to hypoglycemia—a drop in blood sugar. If your glucose falls too low, you might feel weak, drowsy, confused, anxious, or suddenly ravenously hungry.
Is quinine bad for your liver?
When quinine does affect the liver the injury is typically modest. Usually fades away within one to four weeks after the drug is stopped.
How many days is quinine given?
7 days