Novomix 30 Penfill Injection
- Introduction to Novomix 30 Penfill Injection
- Composition and Active Ingredients
- How Novomix 30 Penfill Injection Works
- Novomix30 Uses
- Off-Label Uses of Novomix 30 Penfill Injection
- Dosage and Administration Guidelines for Novomix 30 Penfill
- Special Dosage Considerations
- Storage Instructions for Novomix 30 Penfill Injection
- Novomix 30 side effects
- Other Potential Side Effects of Novomix 30 Penfill Injection
- Drug and Substance Interactions with Novomix 30 Penfill
- Contraindications for Novomix 30 Penfill Use
- Careful Administration and Patient Populations
- Important Precautions While Using Novomix 30 Penfill
- Administration to Elderly Patients
- Administration to Pregnant Women and Nursing Mothers
- Administration to Pediatric Patients
- Overdose and Management Strategies
- Handling Precautions for Novomix 30 Penfill Injection
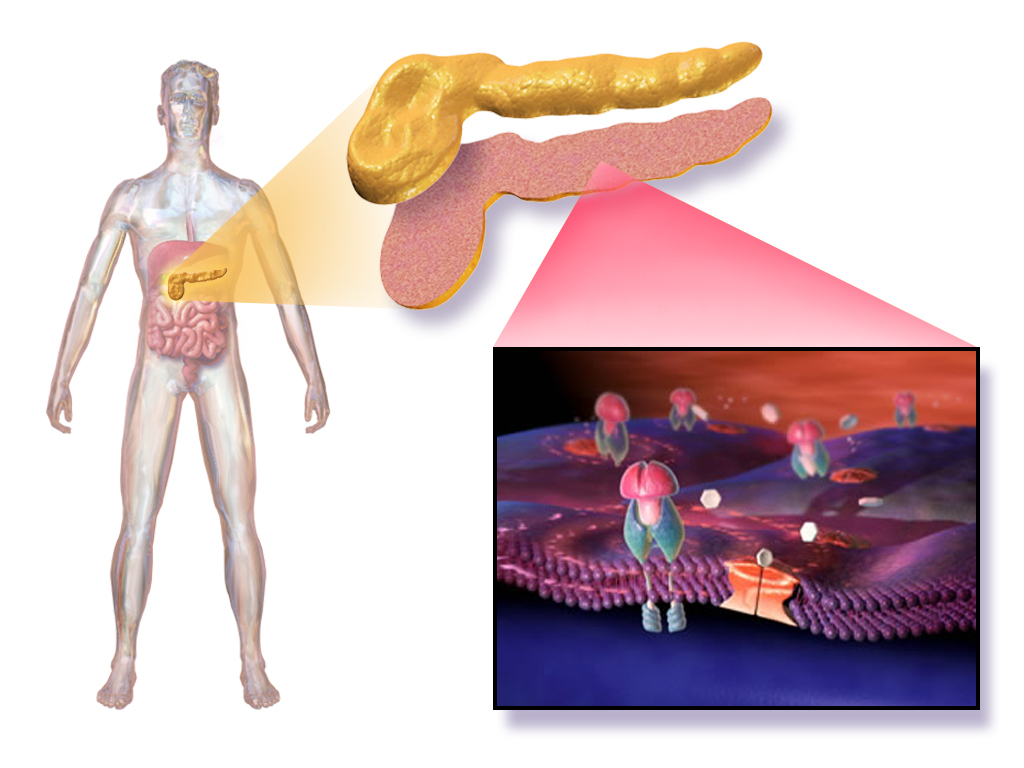
Introduction to Novomix 30 Penfill Injection
Overview of Novomix 30 Penfill
Novomix 30 Penfill Injection is a made insulin mix that aims to regulate blood sugar levels quickly and for an extended period of time by combining two types of insulin aspart to meet the varied needs of people with diabetes mellitus.
Therapeutic Classification and Insulin Type
Novomix 30 falls into the insulin analog category as a medication that stands out for blending rapid and intermediate insulin analogues to better replicate natural insulin reactions in the body.
Importance of Insulin Therapy in Diabetes Management
Insulin therapy remains a cornerstone in diabetes management, especially for patients unable to achieve glycemic targets through lifestyle modifications and oral medications. Novomix 30 helps maintain glycemic equilibrium, minimizing the risks of long-term complications such as nephropathy, retinopathy, and cardiovascular disease.
Composition and Active Ingredients
Detailed Composition of Novomix 30 Penfill
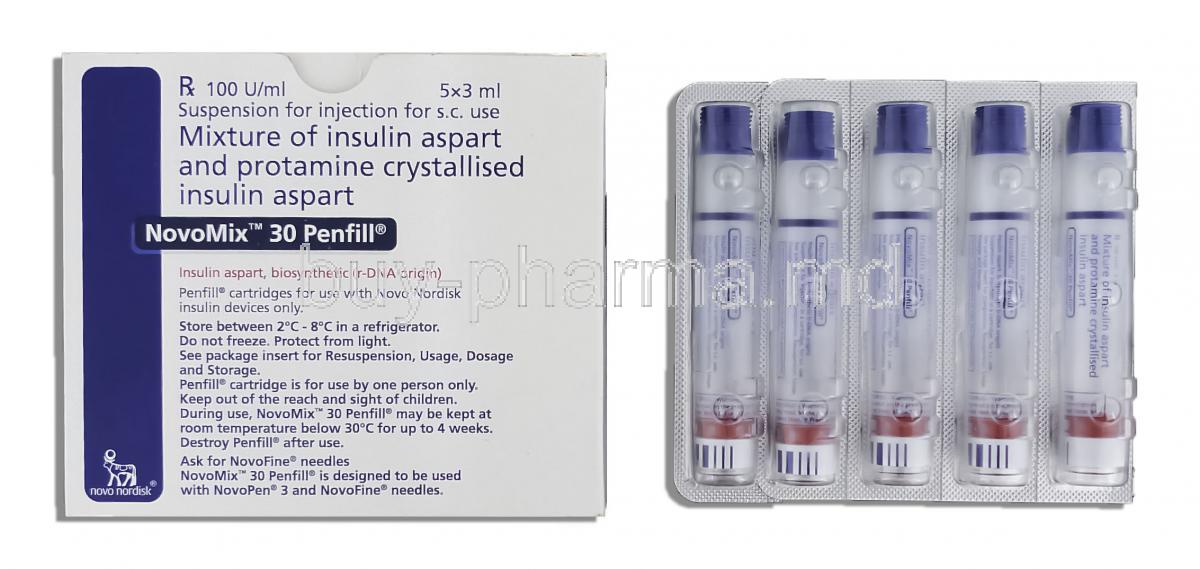
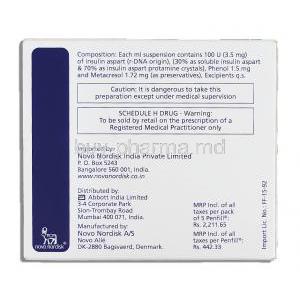
Novomix 30 contains 100 units of insulin aspart in every milliliter. The formulation is 30 percent insulin aspart and 70 percent protamine crystallized insulin aspart suspension.
Insulin Aspart: Rapid-Acting and Intermediate-Acting Components
- Rapid-acting component: Soluble insulin aspart ensures swift onset of glucose regulation post-injection.
- Intermediate-acting component: Protamine-crystallized insulin aspart prolongs the insulin's activity, offering basal glycemic control.
Inactive Ingredients and Their Roles
Additional ingredients include glycerol, phenol, metacresol, zinc chloride, sodium chloride, disodium phosphate dihydrate, and water for injection. These substances stabilize the formulation and ensure its sterility and efficacy.
How Novomix 30 Penfill Injection Works
Mechanism of Action of Insulin Aspart
Novomix 30 duration of action
- Onset: Approximately 10-20 minutes post-injection
- Peak: 1-4 hours after administration
- Duration: Up to 24 hours depending on individual variability
Effect on Glucose Metabolism and Blood Sugar Regulation
Novomix 30 mimics the body's natural insulin response to meals, maintaining baseline glucose levels between meals and thereby reducing both fasting and postprandial blood glucose concentrations.
Novomix30 Uses
Type 1 Diabetes Mellitus: Insulin Replacement Therapy
Novomix 30 is essential for patients with type 1 diabetes, where endogenous insulin production is absent or severely deficient, providing both prandial and basal insulin coverage.
Type 2 Diabetes Mellitus: When Oral Medications Are Insufficient
Combination with Oral Antidiabetic Agents
Novomix 30 can be safely used in conjunction with oral diabetes medications, such as metformin, to enhance blood sugar control without the need for daily injections.
Off-Label Uses of Novomix 30 Penfill Injection
Gestational Diabetes Management

Post-Organ Transplantation Glucose Control
Use in Steroid-Induced Hyperglycemia
Patients receiving long-term corticosteroid therapy may experience steroid-induced diabetes, for which Novomix 30 offers a practical insulin solution.
Use in Critical Care Settings for Tight Glycemic Control
In intensive care settings, Novomix 30 is sometimes utilized for achieving tight glycemic control, essential in reducing morbidity and mortality among critically ill patients.
Dosage and Administration Guidelines for Novomix 30 Penfill
Recommended Starting Dosages and Individualization
Initial dosing should be tailored based on individual needs, typically starting at 0.5-1.0 units/kg/day and adjusted according to blood glucose responses.
Timing of Injection Relative to Meals
Administer Novomix 30 10 minutes before a meal to make sure there is insulin to manage the rise in blood sugar after eating.
Adjustments Based on Blood Glucose Monitoring
Regularly checking your blood sugar levels is important. It's crucial to adjust your medication based on patterns you see, in your fasting and after-meals readings.
Use in Insulin Regimens (Basal-Bolus vs Premixed Protocols)
Novomix 30 can be used in basal-bolus regimens for patients who prefer daily injections and want a simpler, premixed insulin plan.
Special Dosage Considerations
Dose Adjustments During Illness, Surgery, or Stress
Physical stress factors, like illnesses or surgery, can sometimes require increasing the dosage to keep blood sugar levels stable.
Management of Missed Doses
If a dose is forgotten and the next one is coming up soon, it's better to skip the missed dose to prevent blood sugar levels from rising.
Storage Instructions for Novomix 30 Penfill Injection
Recommended Temperature Before First Use
Make sure to keep the Unopened Novomix 30 Penfills in a fridge at a temperature of 2°C to 8 °C and shielded from light.
Storage After Opening: Room Temperature Limits
After first use, the Penfill can be kept at room temperature (below 30°C) for up to 4 weeks away from direct heat and sunlight.
Precautions to Avoid Freezing and Heat Exposure
Freezing damages the insulin structure, rendering it ineffective; therefore, frozen pens must not be used. Exposure to extreme heat must also be avoided.
Shelf Life and Expiration Guidelines
Always adhere to the expiration date on the packaging, and dispose of any insulin that appears cloudy, discolored, or particulate.
Novomix 30 side effects
Injection Site Reactions: Redness, Swelling, Pain
Hypoglycemia: Causes and Symptoms
Hypoglycemia is the most common adverse effect, characterized by symptoms such as trembling, sweating, confusion, and in severe cases, unconsciousness.
Weight Gain Associated with Insulin Therapy
Other Potential Side Effects of Novomix 30 Penfill Injection
Lipodystrophy: Lipoatrophy and Lipohypertrophy
Lipodystrophy refers to localized changes in fat tissue at insulin injection sites. Lipoatrophy involves the atrophy or breakdown of subcutaneous fat, leading to indentations. Conversely, lipohypertrophy manifests as thickened, fatty lumps, which can potentially affect insulin absorption and lead to unpredictable glycemic control.
Hypokalemia and Its Clinical Implications
Insulin therapy can cause potassium to be transported from the bloodstream into cells, which can occasionally result in hypokalemia. Clinical manifestations include muscle weakness, arrhythmias, and, in severe cases, life-threatening cardiac disturbances requiring immediate correction.
Allergic Reactions: Local and Systemic
Localized reactions, like redness and itching at the injection spot, are pretty common, while systemic allergic reactions, such as a full body rash and breathing issues, are rare but can lead to situations, like anaphylaxis, that require medical attention.
Visual Disturbances During Therapy Initiation
Commencement of intensive insulin therapy can temporarily alter vision due to osmotic shifts in the lens of the eye. Although usually transient, patients should be advised to monitor for persistent or worsening symptoms.
Drug and Substance Interactions with Novomix 30 Penfill
Interactions with Oral Antidiabetic Agents
Concomitant use with oral antidiabetics such as sulfonylureas can potentiate the risk of hypoglycemia. Regular blood glucose monitoring is essential to adjust dosing appropriately.
Effects of Beta-Blockers, Corticosteroids, and Thiazide Diuretics
- Beta-blockers: Mask hypoglycemic symptoms like tachycardia, complicating early detection.
- Corticosteroids: Antagonize insulin action, potentially necessitating dosage escalation.
- Thiazide diuretics: Impair glucose tolerance, potentially increasing insulin requirements.
Interaction with Alcohol and Implications for Blood Glucose
Alcohol intake can have unpredictable effects on blood sugar regulation by either increasing the risk of blood sugar or leading to high blood sugar levels based on how much is consumed and one's nutritional status.
Sympathomimetics and Impact on Insulin Action
Some medications, such as decongestants or bronchodilators, may interfere with insulin's function, leading to high blood sugar levels that may require adjusting the insulin dosage temporarily.
Contraindications for Novomix 30 Penfill Use
Hypersensitivity to Insulin Aspart or Excipients
Patients who have a known reaction to insulin aspart or any ingredient in Novomix 30 should avoid using it.
Episodes of Severe Hypoglycemia
Patients experiencing frequent or unawareness of severe hypoglycemia should not initiate therapy until glycemic stability is achieved.
Situations Requiring Discontinuation of Insulin Therapy
Acute illness with significantly reduced oral intake or instances of insulinoma may require reassessment and temporary discontinuation of insulin therapy under medical supervision.
Careful Administration and Patient Populations
Administration Considerations in Patients with Renal Impairment
Extended kidney function can lead to insulin staying in the system longer than usual; therefore, it's essential to be cautious when administering doses and closely monitor patients to prevent fluctuations in blood sugar levels.
Administration Considerations in Patients with Hepatic Impairment
Management Strategies in Patients with Visual or Manual Dexterity Impairments
Patients with vision loss or fine motor challenges should use assisted devices or caregiver support to ensure accurate insulin administration and avoid dosing errors.
Important Precautions While Using Novomix 30 Penfill
Importance of Blood Glucose Self-Monitoring
Regular monitoring is crucial for adjusting insulin doses and avoiding low blood sugar levels by adapting to changes in the body's functions.

Risk Mitigation Strategies for Hypoglycemia
Carrying fast-acting carbohydrates, recognizing early symptoms, and dose adjustments based on activity or meal size are pivotal strategies for hypoglycemia prevention.
Adjustment of Therapy During Intercurrent Illness or Surgery
Illnesses, infections, and surgical procedures can unpredictably alter insulin requirements, demanding closer monitoring and potential dose recalibration.
Administration to Elderly Patients
Dose Adjustments Based on Renal Function
Age-related renal decline mandates cautious titration of insulin dosages, with preference given to lower initial dosing to mitigate hypoglycemia risk.

Increased Risk of Hypoglycemia and Monitoring Recommendations
Older people may show reduced reactions leading to symptoms of low blood sugar but posing a greater risk and needing increased monitoring.
Administration to Pregnant Women and Nursing Mothers
Use of Novomix 30 During Pregnancy: Safety Data
Novomix 30 is typically seen as an option for women to use, and it is suggested to follow intensive insulin therapy to keep blood sugar levels stable and improve the health of the fetus.
Insulin Needs During Different Trimesters
During the three months of pregnancy ( trimester), the need for insulin usually decreases, but increases significantly in the subsequent two trimesters (second and third), requiring frequent dosage adjustments.
Insulin Management During Breastfeeding
Postpartum insulin needs often revert to pre-pregnancy levels. Novomix 30 is compatible with breastfeeding; however, ongoing monitoring is essential to adjust doses appropriately.

Administration to Pediatric Patients
Safety and Efficacy in Children and Adolescents
Novomix 30 has been proven to be safe and effective for children; however, dosage adjustments should be tailored specifically to each child's age, weight, and developmental requirements.
Dosage Individualization in Pediatric Diabetes Management
Children may need their doses adjusted frequently because their insulin sensitivity can change with growth spurts and puberty, as well as their activity levels.
Monitoring Growth and Development Under Insulin Therapy
Consistently checking growth indicators and tracking milestones is crucial to maintaining physical and metabolic well-being while undergoing insulin treatment.
Overdose and Management Strategies
Signs and Symptoms of Insulin Overdose
Symptoms can range from shaking and sweating to heartbeats and mental disorientation in situations where low blood sugar levels can lead to seizures or unconsciousness.
Immediate Interventions for Hypoglycemia
It is crucial to give glucose or intravenous dextrose in cases of emergencies, and administering glucagon injections can also be beneficial for unconscious patients until professional medical assistance is available.
Long-Term Monitoring After Severe Overdose
After experiencing episodes of blood sugar levels (hypoglycemia), it is recommended to closely monitor your blood sugar levels for an extended period to identify any delayed occurrences of low blood sugar or sudden spikes, in high blood sugar (hyperglycemia), and prevent future incidents from happening again.
Handling Precautions for Novomix 30 Penfill Injection
Proper Preparation Before Use
Before administering the solution, visually check for any particles or changes in color. Gently roll the Penfill between your palms to evenly distribute the insulin.
Safe Disposal of Used Penfills and Needles
Make sure to discard used needles and empty Pen Fillers into puncture-resistant sharps containers to avoid any accidental harm and to protect the environment from contamination.
Preventing Contamination and Maintaining Sterility
Remember to follow proper hygiene practices when preparing and administering injections to maintain a sterile environment and prevent infections.
Novomix 30 Penfill Injection FAQ
- What is NovoMix 30 penfill used for?
- What kind of insulin is NovoMix 30?
- When do you inject NovoMix 30?
- Where do you inject NovoMix 30?
- What is the benefit of NovoMix?
- Should NovoMix 30 be given at meal times?
- What are the side effects of NovoMix 30?
- What happens if insulin is taken after food?
- Can NovoMix 30 be given at bedtime?
- How do you use NovoMix 30 penfill?
- Does Novomix insulin cause weight gain?
- Is it okay to skip insulin injection?
- What happens if you take too much insulin by mistake?
- When is the best time to take NovoMix 30?
- What time should a diabetic stop eating at night?
- What are the benefits of NovoMix 30?
- What is NovoMix 30 penfill used for?
- What kind of insulin is NovoMix 30?
- When do you inject NovoMix 30?
- What is the benefit of NovoMix?
- Should NovoMix 30 be given at meal times?
- What happens if insulin is taken after food?
- Can NovoMix 30 be given at bedtime?
- How do you use NovoMix 30 penfill?
What is NovoMix 30 penfill used for?
NovoMix 30 is prescribed to lower blood sugar levels in adults and adolescents, as well as in children aged 10 years and older with diabetes mellitus.
What kind of insulin is NovoMix 30?
A mixture containing 30 percent soluble insulin aspart, a human insulin analogue, and 70 percent protamine crystallized insulin aspart, a human insulin analogue.
When do you inject NovoMix 30?
Right before eating
Where do you inject NovoMix 30?
Abdomen, thighs, buttocks, or upper arms
What is the benefit of NovoMix?
Ensure effective management of sugar levels to regulate blood sugar levels throughout the day.
Should NovoMix 30 be given at meal times?
It's usually best to take it before having a meal.
What are the side effects of NovoMix 30?
Feeling sweaty and trembling with anxiety or shaking; having trouble concentrating or feeling confused; experiencing a heartbeat (palpitations), tingling in the lips; noticing changes in vision; feeling dizzy.
What happens if insulin is taken after food?
Consuming insulin following your meals could increase the chances of experiencing low blood sugar levels, known as hypoglycemia.
Can NovoMix 30 be given at bedtime?
No
How do you use NovoMix 30 penfill?
NovoMix 30 is given through an injection in either the thigh or the abdominal area.
Does Novomix insulin cause weight gain?
Yes
Is it okay to skip insulin injection?
Insufficient insulin intake raises the chances of blood sugar levels (hyperglycemia), leading to long-term diabetes complications.
What happens if you take too much insulin by mistake?
This situation could be quite severe and result in significant hypoglycemia issues. In instances, you might feel disoriented and experience seizures, and in these cases, it could even lead to fatalities.
When is the best time to take NovoMix 30?
NovoMix 30 is usually taken right before eating a meal.
What time should a diabetic stop eating at night?
Ensure that you refrain from eating for approximately 10 to 12 hours every night.
What are the benefits of NovoMix 30?
NovoMix includes two types of insulin aspart. One that starts working within 10 minutes of injection and another that is absorbed slowly throughout the day in crystallized form. The substitute insulin functions similarly to naturally occurring insulin by aiding in the entry of glucose into cells from the bloodstream.
What is NovoMix 30 penfill used for?
NovoMix 30 is prescribed for lowering blood sugar levels in adults and young individuals aged 10 years and older who have diabetes mellitus (diabetes).
What kind of insulin is NovoMix 30?
A mixture comprising 30 percent soluble insulin aspart, which acts rapidly like insulin, and 70 percent protamine crystallized insulin aspart with action akin to human insulin.
When do you inject NovoMix 30?
Just before eating or whenever needed after starting a meal.
What is the benefit of NovoMix?
Make sure to maintain swift sugar management to regulate blood sugar levels throughout the day.
Should NovoMix 30 be given at meal times?
It's usually best to take it just before eating.
What happens if insulin is taken after food?
Having your insulin after you eat could increase the chances of experiencing low blood sugar levels or hypoglycemia.
Can NovoMix 30 be given at bedtime?
No
How do you use NovoMix 30 penfill?
NovoMix 30 is typically administered through an injection, under the skin, either in the thigh or abdomen.