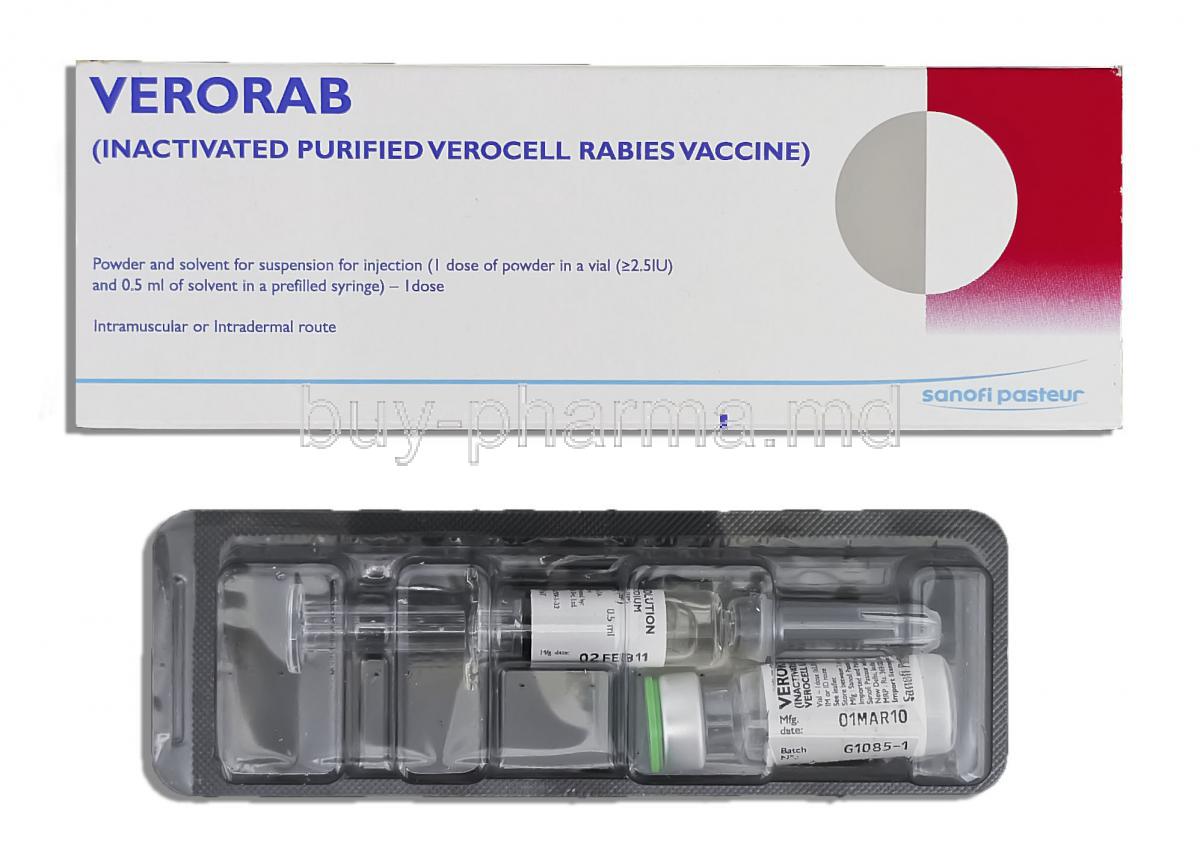
1. Introduction to Verorab Rabies Vaccine
Verorab is a purified, inactivated rabies vaccine derived from Vero cell cultures. Developed using advanced virological techniques, it ensures high immunogenicity and a strong safety profile. As a cell culture–based rabies vaccine, it eliminates the risks associated with older nerve tissue–derived vaccines.
Manufactured by Sanofi, Verorab is globally distributed and widely used in public health systems, private clinics, and travel medicine programs. It is listed as an essential medicine by the World Health Organization (WHO), underlining its critical role in the prevention and control of rabies in both endemic and non-endemic regions.
Its primary indications include:
- Pre-exposure prophylaxis for individuals at risk of rabies due to occupation or travel
- Post-exposure prophylaxis following bites or scratches by animals suspected of rabies
WHO endorses Verorab for use in rabies immunization programs as part of both intramuscular and intradermal schedules, especially in regions with limited access to healthcare.
2. Medical and Preventive Uses of Verorab
2.1 Approved Uses
Verorab is approved for two major clinical scenarios:
- Pre-exposure prophylaxis (PrEP): Recommended for veterinarians, animal handlers, laboratory workers handling rabies virus, and travelers to high-risk areas. It ensures protection prior to any potential rabies exposure.
- Post-exposure prophylaxis (PEP): Indicated following potential rabies exposure, including:
- Animal bites or scratches
- Mucosal contamination from infected saliva
- Open wounds exposed to potentially rabid animals
2.2 Off-Label Uses and Investigational Applications
In some cases, Verorab may be used outside standard labeling:
- Administration to immunocompromised patients with altered dosing or scheduling
- Use in patients with interrupted or incomplete vaccination series
- Emergency mass vaccination programs in areas facing rabies outbreaks or natural disasters disrupting healthcare access
3. Mechanism of Action: How Verorab Works
Verorab introduces purified inactivated rabies virus antigen, specifically targeting the rabies glycoprotein G, which is essential for virus entry into host cells.
- The immune system responds by producing virus-neutralizing antibodies
- These antibodies prevent the virus from reaching the central nervous system
- Protective levels are typically reached within 7–14 days post-vaccination
In PEP cases, Verorab is administered alongside Rabies Immune Globulin (RIG) to provide immediate passive immunity while active immunization builds over time.
4. Verorab Composition and Formulation
- Active Ingredient: Inactivated rabies virus (Wistar strain PV-BHK21)
- Production method: Grown in Vero cell cultures, then purified and inactivated using β-propiolactone
- Excipients: Includes buffer solutions, stabilizers, and trace antibiotics (e.g., neomycin)
- Presentation: Lyophilized powder in a glass vial with a separate vial of diluent
Each reconstituted dose delivers a minimum of 2.5 IU of rabies antigen, suitable for both intramuscular and intradermal administration.
5. Dosage and Administration Guidelines
5.1 Pre-Exposure Prophylaxis Protocols
The standard intramuscular regimen consists of three doses:
- Day 0, Day 7, and Day 21 or 28
Intradermal protocols are also endorsed by WHO and may involve lower antigen doses, especially in resource-limited settings. Booster doses are advised every 3–5 years for continuous-risk individuals, or after potential exposure if antibody titers are low.
5.2 Post-Exposure Prophylaxis (PEP) Protocol
Two main IM schedules are recommended:
- Essen protocol: 5 doses on Days 0, 3, 7, 14, and 28
- Zagreb protocol: 2 doses on Day 0 (both deltoids), followed by single doses on Days 7 and 21
RIG should be co-administered on Day 0 for category III exposures, infiltrated around the wound and injected intramuscularly elsewhere if needed.
5.3 Catch-Up Dosing and Incomplete Schedule Management
If doses are delayed or skipped:
- Continue remaining doses without restarting the entire series
- Consult serological testing if immunosuppression or uncertainty exists
6. Common and Serious Side Effects of Verorab
6.1 Common Adverse Reactions
- Injection site pain, redness, or induration
- Fever, headache, fatigue, or muscle aches
- Gastrointestinal discomfort in some cases
Most symptoms resolve within 1–2 days and do not require treatment.
6.2 Less Common but Serious Reactions
- Hypersensitivity reactions: rash, urticaria, or angioedema
- Anaphylactic shock (very rare)
- Neurological complications like Guillain-Barré syndrome (extremely rare)
All serious events must be reported to pharmacovigilance authorities promptly.
7. Warnings, Contraindications, and Precautions
7.1 General Warnings
- Verorab does not replace immediate and thorough wound cleansing
- Incomplete vaccination may result in inadequate immunity
- Immunosuppressive treatments can reduce vaccine effectiveness
7.2 Contraindications
- Known allergy to any component of the vaccine
- History of anaphylaxis after previous rabies vaccination
7.3 Important Precautions Before Use
- Review of past allergic reactions or immunodeficiencies
- Monitoring of patient for at least 30 minutes post-injection
- Appropriate resuscitation equipment should be accessible
8. Drug and Vaccine Interactions with Verorab
- Immunosuppressants (e.g., chemotherapy, biologics) may blunt antibody response
- High-dose corticosteroids can delay seroconversion
- Concurrent administration with other vaccines is permitted but should be injected at separate sites
Inactivated vaccines like Verorab generally do not interfere with other vaccines but spacing is recommended when possible.
9. Handling, Storage, and Disposal Guidelines
9.1 Proper Storage Conditions
Verorab must be stored under strict cold chain conditions to maintain its immunogenic potency. The vaccine should be kept refrigerated at a controlled temperature between 2°C and 8°C at all times. Freezing must be strictly avoided, as it can irreversibly denature the vaccine’s active components and render it ineffective.
Each vial should be inspected regularly for any signs of discoloration or particulate matter. Healthcare facilities should implement a rotation system, ensuring that older stock is used first and that expiration dates are routinely checked. Products should be discarded if the expiration date is exceeded or if there is evidence of improper storage.
9.2 Reconstitution and Handling Instructions
Verorab is supplied as a lyophilized powder that must be reconstituted using the sterile diluent provided with each vial. The following steps are critical to ensure correct preparation:
- Draw the entire contents of the diluent vial into a sterile syringe.
- Inject the diluent into the vial containing the freeze-dried vaccine powder.
- Gently swirl the vial to mix; avoid vigorous shaking.
- Use the reconstituted vaccine within 30 minutes to avoid loss of potency.
All needles and syringes must be handled with care to prevent accidental injury or contamination. Single-use devices should never be reused. After administration, used sharps must be immediately placed in puncture-proof sharps containers in accordance with biomedical waste management protocols.
9.3 Disposal of Expired or Unused Vaccine
Expired or unused vaccine vials should be disposed of as per national medical waste disposal guidelines. These typically include:
- Labeling and segregating biological waste separately from general waste
- Autoclaving or incinerating expired vials in approved medical waste facilities
- Documenting the batch number, quantity, and reason for disposal for regulatory compliance
Proper disposal prevents environmental contamination and accidental use of compromised vaccine material.
10. Overdose and Emergency Management
Overdose with Verorab is uncommon, but may occur due to incorrect administration or repeated dosing outside of the recommended schedule. Instances may include administration of an extra dose or re-administration of a full course due to uncertainty in exposure history.
Symptoms following overdose are generally mild and include localized pain, swelling, or systemic symptoms like fever or fatigue. Severe outcomes are rare due to the vaccine’s high safety profile.
In such cases:
- Symptomatic treatment should be initiated
- The patient should be observed for adverse effects, especially hypersensitivity
- No specific antidote exists; management is supportive and primarily observational
11. Guidelines for Careful Administration in Special Populations
11.1 Use in Elderly Patients
In geriatric patients, immunosenescence may result in a reduced or delayed immune response. While Verorab remains safe and well-tolerated in the elderly, clinicians should monitor these patients more closely to assess vaccine efficacy and side effect profile.
Common concerns in this demographic include:
- Subtle adverse events that may be misattributed to underlying comorbidities
- Need for booster dose evaluation based on antibody titers
11.2 Use in Pregnant and Lactating Women
Verorab is considered safe for administration during pregnancy and lactation. WHO supports its use when the risk of rabies exposure outweighs potential risks to the mother or fetus. The vaccine does not contain live virus and therefore does not pose a teratogenic threat.
Key considerations include:
- Assessing the exposure risk in rabies-endemic regions
- Avoiding delay in PEP in pregnant women following exposure
- Compatibility with breastfeeding—no need to interrupt nursing
11.3 Use in Pediatric Populations
Verorab is approved for use in children, including infants. The dosage is the same as for adults, though administration technique may differ based on age.
Special precautions for children include:
- Using the anterolateral thigh for intramuscular injections in infants under 2 years
- Providing distraction or comfort measures during vaccination
- Monitoring for fever and local reactions, which may be more pronounced
12. Safe Handling Precautions for Healthcare Workers and Caregivers
Personnel involved in the storage, preparation, and administration of Verorab must adhere to strict biosafety and infection control protocols. The following precautions are essential:
- Use of personal protective equipment (PPE), including gloves and lab coats
- Hand hygiene before and after handling the vaccine
- Proper disposal of reconstitution equipment and syringes in sharps containers
In the event of accidental exposure (e.g., needlestick injury or splash to mucous membranes), the affected individual should:
- Immediately wash the area with soap and water or flush with saline
- Report the incident and seek medical evaluation
- Follow institutional protocols for post-exposure assessment
Stringent training and vigilance help prevent occupational exposure and ensure safe vaccine handling throughout the immunization process.
Verorab Rabies Vaccine FAQ
- How many doses of Verorab vaccine are given?
- How effective is the Verorab vaccine?
- Do you need 2 or 3 doses of rabies vaccine?
- What is the generic name of Verorab?
- How do you inject Verorab vaccine?
- What are the side effects of verorab?
- Can I stop rabies vaccine after 3 doses?
- How many rabies shots if bitten?
- What to avoid after anti-rabies vaccine?
- What to eat after rabies vaccine?
- How many shots do you need to survive rabies?
- Is it okay to change the anti-rabies vaccine?
- What is the schedule for the verorab rabies vaccine?
- How do you inject Verorab?
- How long is the anti-rabies vaccine effective in humans?
- Are you fully protected from rabies once vaccinated?
- How many hours should I get anti rabies shot after being bitten?
- How many doses of verorab are given?
How many doses of Verorab vaccine are given?
The suggested pre-exposure prophylaxis plan involves receiving three vaccine shots on days 0, 7, and 21 or 28, through either injection into the muscle or under the skin.
How effective is the Verorab vaccine?
Seroconversion rates of 90–100%, indicating the presence of rabies virus neutralizing antibodies (RVNA), were documented within weeks, regardless of the treatment plan used; furthermore, strong booster reactions were observed after 1 year (with all participants showing seroconversion rates of 100% by day 14 after receiving the booster shot).
Do you need 2 or 3 doses of rabies vaccine?
The rabies shot is administered through an injection in three doses, spread out over 28 days. However, if there is a time constraint, the doses can also be given over 21 days or sometimes over 7 days.
What is the generic name of Verorab?
Inactivated Rabies Vaccine (Derived from Wistar Strain Rabies Virus)
How do you inject Verorab vaccine?
The vaccine is given through a shot, in the muscle of the arm for adults and children, or in the area of the thigh muscle for infants and toddlers.
What are the side effects of verorab?
Common side effects often experienced include feeling under the weather, headaches, muscle soreness, pain, redness, or swelling at the injection site. Additionally, in infants; irritability, crying and drowsiness.
Can I stop rabies vaccine after 3 doses?
You might consider halting the vaccination process after the dose given on day 7.
How many rabies shots if bitten?
Four doses of the rabies vaccine are administered over a 14-day period.
What to avoid after anti-rabies vaccine?
After an anti-rabies vaccine, you should avoid alcoholic drinks, raw or undercooked food, and foods with high sugar content
What to eat after rabies vaccine?
A rounded diet full of foods, like fruits and vegetables, along with lean meats and whole grains, is essential for good health.
How many shots do you need to survive rabies?
Four injections over 14 days
Is it okay to change the anti-rabies vaccine?
It's completely safe and acceptable to switch to a different rabies vaccine brand to complete your series of doses.
What is the schedule for the verorab rabies vaccine?
3 doses Days 0, 7, and 21 or 28
How do you inject Verorab?
Verorab should not be administered in the buttocks area. The vaccine should not be given through the bloodstream route.
How long is the anti-rabies vaccine effective in humans?
1 to 2 years
Are you fully protected from rabies once vaccinated?
Ninety-five percent of individuals who receive three doses of the rabies vaccine will experience some level of protection against rabies. The duration of this protection can typically last for a minimum of one to two years.
How many hours should I get anti rabies shot after being bitten?
Preferably within 24 hours, but certainly within 72 hours
How many doses of verorab are given?
4