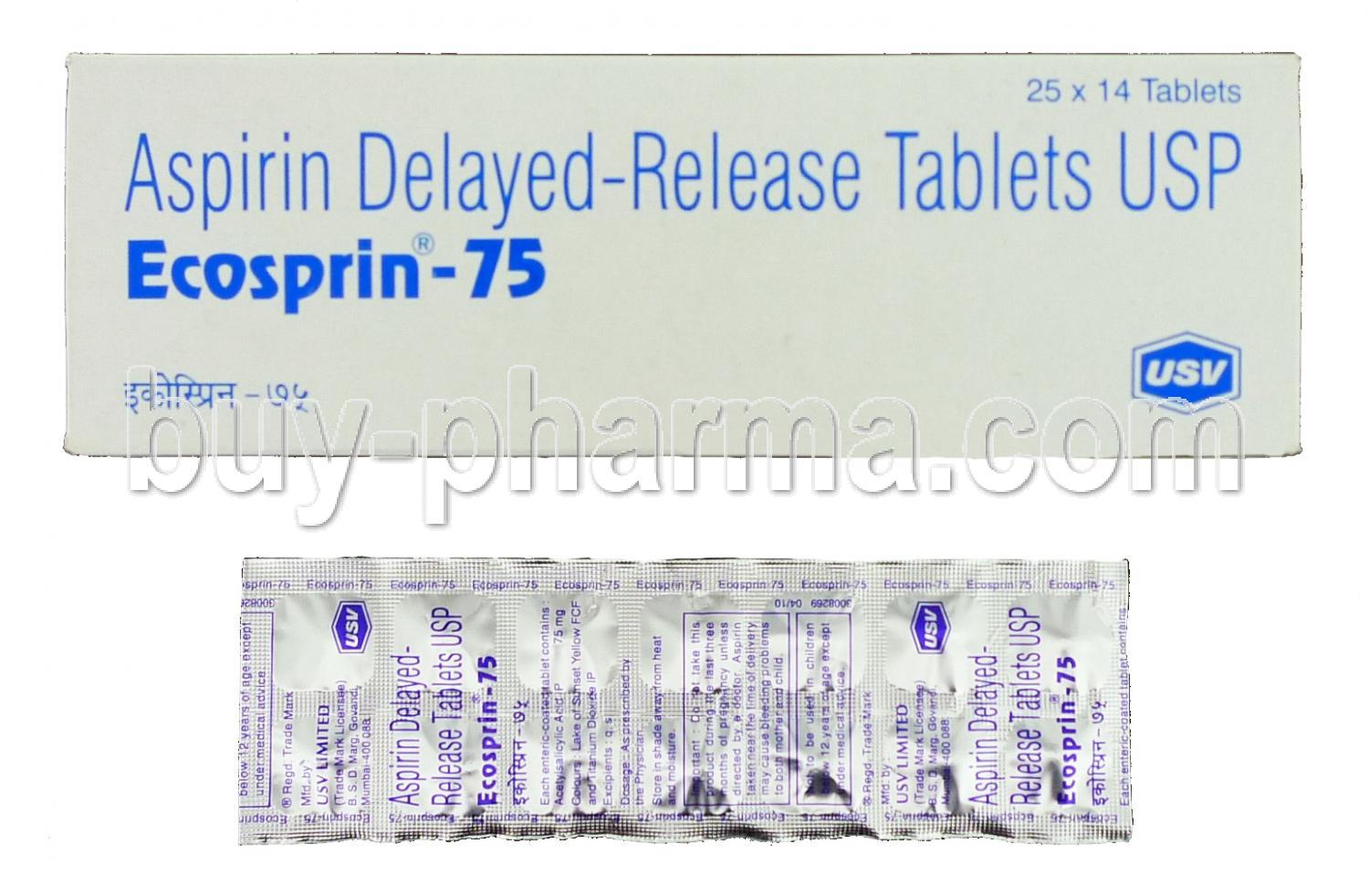
Introduction to Ecosprin (Aspirin)
Ecosprin is a widely prescribed pharmaceutical containing acetylsalicylic acid, commonly known as aspirin. Recognized for its cardio-protective and anti-inflammatory properties, Ecosprin plays a pivotal role in the prevention of cardiovascular events and the management of pain and fever.
This medication belongs to two pharmacological classes: non-steroidal anti-inflammatory drugs (NSAIDs) and antiplatelet agents. Its dual-action profile makes it suitable for a variety of clinical indications.
While Ecosprin and generic aspirin share the same active ingredient, Ecosprin is specifically formulated with enteric coating to minimize gastric irritation and ensure targeted release. It is typically available in multiple strengths, including Ecosprin 75 mg, 150 mg, and 325 mg, catering to different therapeutic needs.
Historically, aspirin has been used for over a century. Ecosprin is a modern, optimized version developed for enhanced tolerability in long-term therapies, especially in cardiac patients.
Composition and Active Ingredients
The principal component of Ecosprin is acetylsalicylic acid, a synthetic derivative of salicylic acid. This compound exerts its effect by modulating prostaglandin synthesis.
Each tablet may also contain pharmaceutical excipients such as maize starch, microcrystalline cellulose, and hypromellose. The presence of enteric coating distinguishes Ecosprin from uncoated aspirin tablets, reducing direct gastric mucosal contact and thereby mitigating gastrointestinal side effects.
Ecosprin is considered a first-line antiplatelet agent. In comparison to clopidogrel, another popular antiplatelet, Ecosprin offers cost-effective primary and secondary prophylaxis in cardiovascular care.
Pharmacological Mechanism of Action
Ecosprin acts by irreversibly inhibiting cyclooxygenase enzymes (COX-1 and COX-2), which are pivotal in the conversion of arachidonic acid to thromboxanes and prostaglandins.
- COX-1 inhibition leads to decreased thromboxane A2 production, suppressing platelet aggregation.
- COX-2 inhibition contributes to anti-inflammatory and analgesic effects.
Due to its irreversible binding to COX enzymes, Ecosprin's antiplatelet effect lasts for the entire lifespan of platelets (7–10 days), even though its plasma half-life is only 15–20 minutes. Effects generally begin within 30 minutes to 2 hours post-ingestion.
Approved Medical Uses of Ecosprin
4.1 Cardiovascular Indications
- Myocardial infarction prevention: Used as a daily low-dose therapy to prevent heart attacks in at-risk individuals.
- Secondary prevention of stroke and TIA: Maintains blood flow in cerebral vessels by reducing clot formation.
- Post-angioplasty and stent placement: Essential component of dual antiplatelet therapy (DAPT) with clopidogrel.
- Unstable angina and acute coronary syndrome: Administered as an emergency intervention and long-term prevention strategy.
4.2 Antiplatelet Use in High-Risk Populations
- Patients with diabetes mellitus, hypertension, or metabolic syndrome often benefit from long-term low-dose Ecosprin therapy.
- Peripheral arterial disease (PAD) management includes antiplatelet therapy to improve limb perfusion and reduce thrombotic risk.
4.3 Pain and Inflammation Management
Ecosprin may be used in higher doses for analgesia and inflammation control, especially in:
- Osteoarthritis and rheumatoid arthritis
- Acute musculoskeletal injuries or overuse syndromes
- Post-operative or dental pain management
4.4 Fever and Headache Relief
As an antipyretic, Ecosprin effectively reduces fever caused by viral or bacterial infections. It is also utilized for:
- Tension headaches and migraines
- Febrile illnesses in adults (with caution)
Off-Label and Investigational Uses
- Colorectal cancer prevention: Emerging evidence supports aspirin’s role in reducing adenoma and carcinoma risk in high-risk individuals.
- Preeclampsia prevention: Low-dose Ecosprin is recommended in pregnant women with a high risk of preeclampsia.
- Venous thromboembolism (VTE) prevention: Used off-label in orthopedic post-surgical prophylaxis.
- Kawasaki disease: In pediatric care, aspirin is used for its antiplatelet and anti-inflammatory effects.
Dosage and Administration Guidelines
6.1 Recommended Dosage by Indication
- Cardiovascular prophylaxis: 75–150 mg once daily
- Inflammatory and pain management: 325–650 mg every 4–6 hours, not exceeding 4 g/day
- Antipyretic use: 325–500 mg every 6 hours as needed
6.2 Administration Instructions
- Best taken after meals to reduce gastrointestinal irritation
- Enteric-coated tablets must not be crushed or chewed
- If a dose is missed, take it as soon as remembered unless it’s almost time for the next dose
- In case of overdose, immediate medical attention is required
Common and Serious Side Effects
7.1 Common Side Effects
- Gastric discomfort, nausea, vomiting
- Heartburn, dyspepsia
- Minor bruising or bleeding due to platelet inhibition
- Tinnitus, especially at high doses
7.2 Less Common and Serious Adverse Effects
- Gastrointestinal bleeding, peptic ulcer perforation
- Hemorrhagic stroke or intracranial bleeding
- Hypersensitivity reactions, bronchospasm
- Reye’s syndrome in pediatric populations exposed to aspirin during viral illness
Drug Interactions
- Anticoagulants: Enhanced bleeding risk when combined with warfarin, heparin
- Other NSAIDs or corticosteroids: Increased gastrointestinal toxicity
- ACE inhibitors and beta-blockers: Reduced antihypertensive efficacy
- Alcohol: Additive effect on gastrointestinal bleeding
- Methotrexate and lithium: Aspirin can elevate serum levels, increasing toxicity
Contraindications
- History of peptic ulcer disease or gastrointestinal bleeding
- Known allergy to aspirin or other NSAIDs
- Bleeding disorders such as hemophilia
- Active liver disease, especially with coagulopathy
- Severe renal impairment (eGFR <30 mL/min/1.73 m²)
- Pediatric patients with viral infections due to risk of Reye’s syndrome
Warnings and Precautions
When administered over prolonged periods, Ecosprin can significantly elevate the risk of gastrointestinal bleeding, particularly in patients with a history of ulcers, gastritis, or concurrent NSAID use. This risk is further amplified in individuals who consume alcohol regularly or smoke.
Patients with asthma or nasal polyps may experience exacerbation of respiratory symptoms due to aspirin-exacerbated respiratory disease (AERD). In such cases, medical supervision is critical.
Abrupt discontinuation of Ecosprin, particularly in patients with established cardiovascular disease, may provoke thrombotic rebound events. Sudden cessation can precipitate myocardial infarction or ischemic stroke. A tapering strategy should be implemented when discontinuation is deemed necessary.
In undernourished or low-body-weight individuals, aspirin metabolism and toxicity thresholds may differ. A meticulous risk-benefit evaluation is essential before initiating long-term therapy.
Guidelines for Careful Administration
Patients with chronic hepatic or renal impairment require vigilant monitoring when prescribed Ecosprin. The drug’s excretion and metabolism are dependent on hepatic enzymes and renal clearance, making accumulation and toxicity more likely in compromised systems.
For those at high risk of gastrointestinal bleeding, co-administration of gastroprotective agents such as proton pump inhibitors (PPIs) or H2-receptor antagonists is advised.
Routine evaluation of hemoglobin levels and fecal occult blood tests should be incorporated into follow-up visits, particularly in patients on high-dose or long-term therapy. These tests help detect occult gastrointestinal bleeding early.
Special Populations
12.1 Use in Elderly Patients
The geriatric population exhibits increased gastrointestinal mucosal sensitivity, predisposing them to ulceration and hemorrhage with prolonged aspirin use. Renal function also tends to decline with age, affecting drug clearance.
Age-related physiological changes contribute to a higher incidence of falls and complications from bleeding events, including intracranial hemorrhage. Therefore, careful dose adjustment and periodic monitoring of coagulation parameters, renal function, and hemoglobin are strongly recommended.
12.2 Use During Pregnancy and Lactation
Ecosprin is contraindicated in the third trimester of pregnancy due to its ability to cause premature closure of the ductus arteriosus in the fetus, leading to pulmonary hypertension and fetal distress.
In the first and second trimesters, use is restricted to specific high-risk scenarios, as aspirin is associated with an increased risk of miscarriage, fetal renal dysfunction, and intrauterine growth retardation.
Although only minimal quantities of aspirin are excreted in breast milk, its use during lactation should be approached with caution, particularly in neonates or premature infants, due to their immature metabolic pathways.
12.3 Use in Pediatric Patients
Aspirin is strictly contraindicated in children and adolescents with viral infections due to the risk of Reye’s syndrome—a potentially fatal condition involving acute encephalopathy and hepatic dysfunction.
However, in specific conditions like Kawasaki disease, Ecosprin is administered under specialist care for both anti-inflammatory and antiplatelet effects. Pediatric dosing must be carefully calculated based on weight and clinical need, with close monitoring for toxicity.
Overdose and Emergency Management
Signs of acute salicylate toxicity include:
- Metabolic acidosis with increased anion gap
- Hyperventilation and respiratory alkalosis
- Tinnitus, confusion, hyperthermia, and vomiting
Immediate management begins with the administration of activated charcoal if the ingestion was recent. Supportive care includes:
- Intravenous fluid resuscitation to correct dehydration and electrolyte imbalance
- Urinary alkalinization using sodium bicarbonate to enhance renal salicylate excretion
- Hemodialysis in severe cases, especially when renal failure, severe acidosis, or altered mental status is present
Prompt recognition and intervention are vital to prevent irreversible systemic damage or death.
Storage and Handling Precautions
Ecosprin should be stored at a controlled room temperature, ideally below 25°C, in a dry place protected from moisture and direct sunlight. Humidity can compromise tablet integrity and reduce therapeutic efficacy.
To ensure safety, Ecosprin must be kept out of reach of children at all times, as accidental ingestion can lead to serious complications.
Expired tablets should not be consumed and must be disposed of responsibly in accordance with local pharmaceutical waste regulations. Do not flush or throw them in household trash.
Signs of tablet degradation include discoloration, unusual odor, or crumbling of the tablet surface—such tablets should not be used.